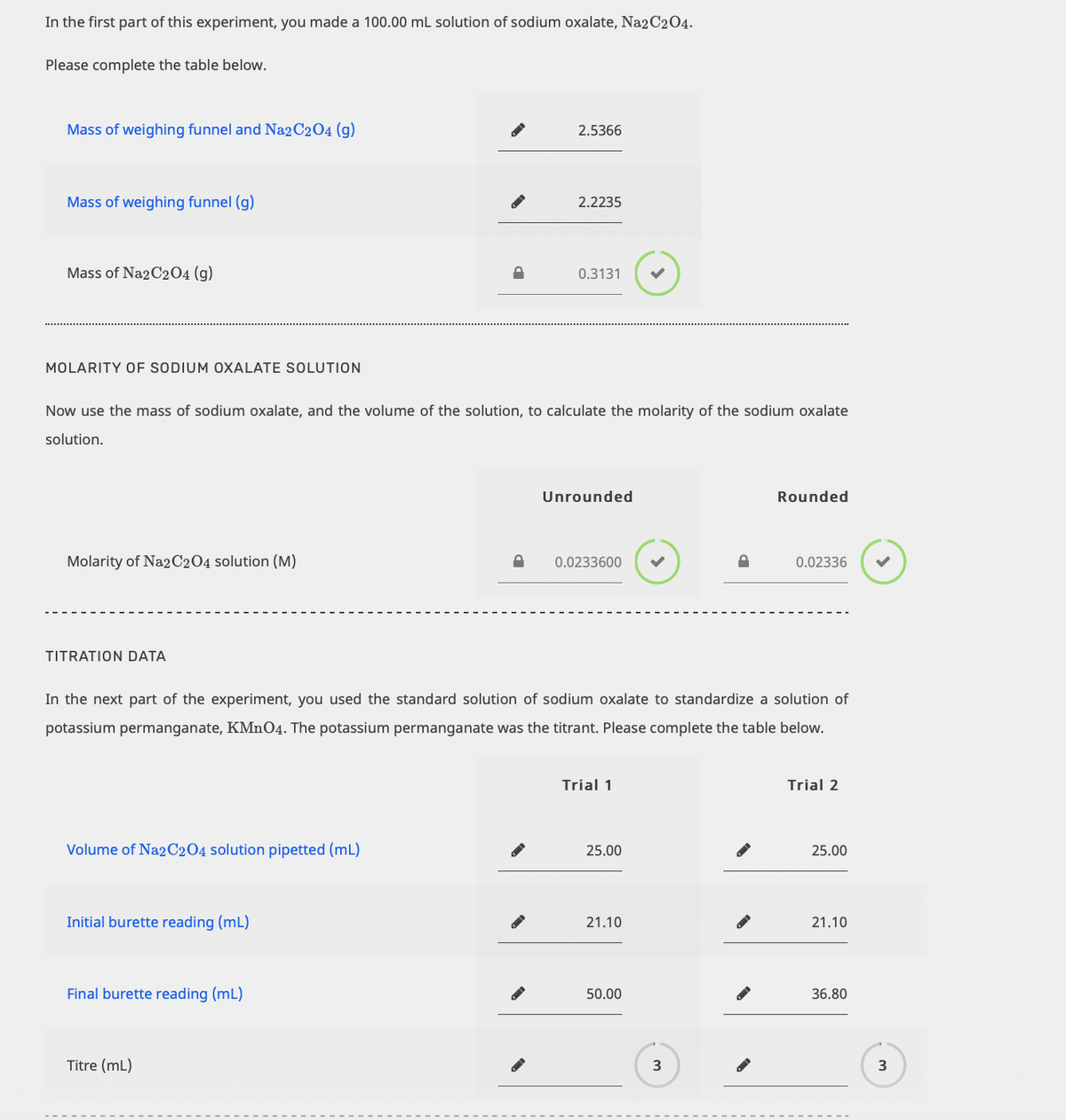
In the next part of the experiment, you used the standard solution of sodium oxalate to standardize a solution of potassium permanganate, KMN04. The potassium permanganate was the titrant. Please complete the table below. Trial 1 Trial 2 Volume of Na2C2O4 solution pipetted (mL) 25.00 25.00 Initial burette reading (mL) 21.10 21.10 Final burette reading (mL) 50.00 36.80 Titre (mL) 3
In the next part of the experiment, you used the standard solution of sodium oxalate to standardize a solution of potassium permanganate, KMN04. The potassium permanganate was the titrant. Please complete the table below. Trial 1 Trial 2 Volume of Na2C2O4 solution pipetted (mL) 25.00 25.00 Initial burette reading (mL) 21.10 21.10 Final burette reading (mL) 50.00 36.80 Titre (mL) 3
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 4ALQ
Related questions
Question
I need help calculating the "titre" for both trials please (shown in the first picture).
Transcribed Image Text:In the first part of this experiment, you made a 100.00 mL solution of sodium oxalate, Na2C2O4.
Please complete the table below.
Mass of weighing funnel and Na2C204 (g)
2.5366
Mass of weighing funnel (g)
2.2235
Mass of Na2C2O4 (g)
0.3131
MOLARITY OF SODIUM OXALATE SOLUTION
Now use the mass of sodium oxalate, and the volume of the solution, to calculate the molarity of the sodium oxalate
solution.
Unrounded
Rounded
Molarity of Na2C2O4 solution (M)
0.0233600
0.02336
TITRATION DATA
In the next part of the experiment, you used the standard solution of sodium oxalate to standardize a solution of
potassium permanganate, KMNO4. The potassium permanganate was the titrant. Please complete the table below.
Trial 1
Trial 2
Volume of Na2C2O4 solution pipetted (mL)
25.00
25.00
Initial burette reading (mL)
21.10
21.10
Final burette reading (mL)
50.00
36.80
Titre (mL)

Transcribed Image Text:Part 1. Standardization of the KMN0, Solution
Experimental Data
Mass of weighing funnel and Na,C,O, 2.5366g
2.2235g
Mass of weighing funnel
Titrations
Titration #1
Titration #2
Volume of Na,C,O, pipetted
25.00mL
22.3mL
Initial burette reading
21,1 mL
22.3mL
Final burette reading
50.1mL
36.8mL
(value provided by TA,
on the board)
Zone average molarity of KMnO,
0,01366 M
Reaction
2 MnO, + 5 C,0, + 16 H* → 2 Mn2+ + 10 CO, + 8 H,O
Calculated Values
Mass of Na,C,O4
03131g
(Give to Lab TA)
Molarity of Na,C,O,
Titrations
Titration #1
Titration #2
28.0mL
1.45 mL
Volume of KMNO,
(Give to Lab TA)
solution
Molarity of KMnO,
Average molarity of KMNO,
% deviation of your molarity of
KMNO, from zone average
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning