In the Periodic Table below, shade all the elements for which the neutral atom has an outer electron configuration of ms nd", where n and m are integers, and m=n+1.
In the Periodic Table below, shade all the elements for which the neutral atom has an outer electron configuration of ms nd", where n and m are integers, and m=n+1.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter2: Atomic Structure And Periodicity
Section: Chapter Questions
Problem 146AE: An unknown element is a nonmetal and has a valence electron configuration of ns2np4 a. How many...
Related questions
Question
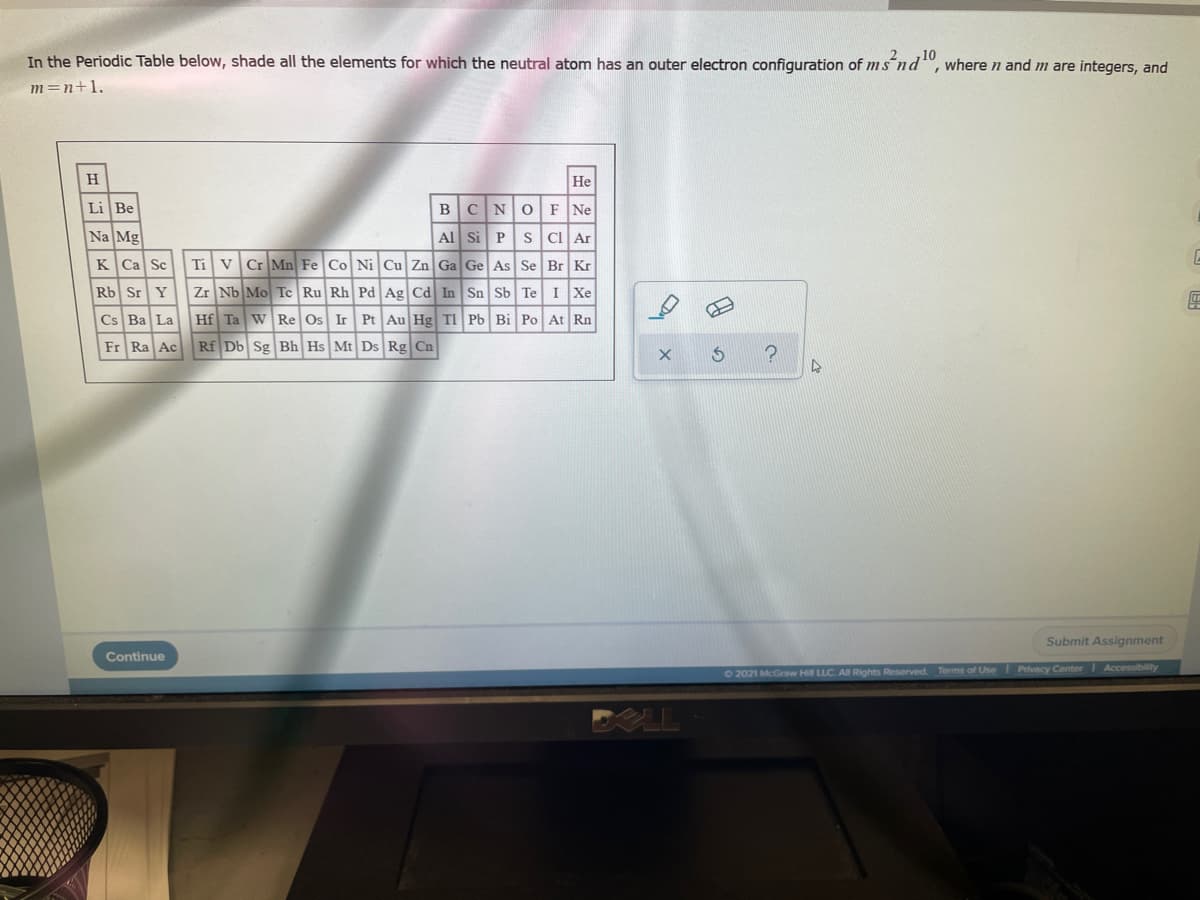
Transcribed Image Text:In the Periodic Table below, shade all the elements for which the neutral atom has an outer electron configuration of ms nd", where n and m are integers, and
m=n+1.
H
He
Li Be
BCNOF Ne
Na Mg
Al Si PS CI Ar
Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr
Zr Nb Mo Te Ru Rh Pd Ag Cd In Sn Sb Te I Xe
Cs Ba La Hf Ta W Re Os Ir Pt Au Hg TI Pb Bi Po At Rn
K Ca Sc
Rb Sr Y
Fr Ra Ac
Rf Db Sg Bh Hs Mt Ds Rg Cn
Submit Assignment
Continue
O2021 McGraw Hil LLC. A Rights Reserved. Terms of UseI Privacy Center Accessibility
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning