In the presence of a small amount of bromine, cyclohexene undergoes the following light-promoted reaction: Br hv + trace Br2 + НBr cyclohexene 3-bromocyclohexene Part A Propose a mechanism for this reaction. Draw all missing reactants and/or products in the appropriate boxes by placing atoms on the canvas and connecting them with bonds. Add charges where needed. Electron flow arrows should start on the electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created. Include all free radicals by rightclicking on an atom on the canvas and then using the Atom properties to select the monovalent radical. H :Br +H CI Br Br i O do A mechanism must contain at least two rectangles, one containing starting materials and the other products. No credit lost. Try again. 1 0 +1•
In the presence of a small amount of bromine, cyclohexene undergoes the following light-promoted reaction: Br hv + trace Br2 + НBr cyclohexene 3-bromocyclohexene Part A Propose a mechanism for this reaction. Draw all missing reactants and/or products in the appropriate boxes by placing atoms on the canvas and connecting them with bonds. Add charges where needed. Electron flow arrows should start on the electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created. Include all free radicals by rightclicking on an atom on the canvas and then using the Atom properties to select the monovalent radical. H :Br +H CI Br Br i O do A mechanism must contain at least two rectangles, one containing starting materials and the other products. No credit lost. Try again. 1 0 +1•
Chapter16: Chemistry Of Benzene: Electrophilic Aromatic Substitution
Section16.SE: Something Extra
Problem 30MP: The carbocation electrophile in a Friede1-Crafts reaction can be generated by an alternate means...
Related questions
Question
For the first part can you have the same format as the one from the photo. And the second I need help drawing the structure. What do they mean use rectangles?
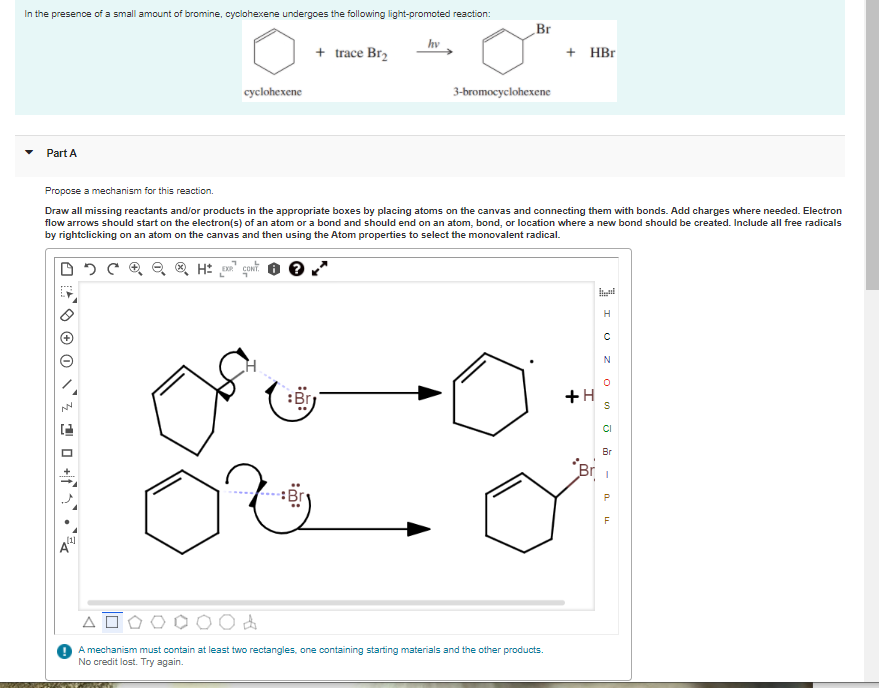
Transcribed Image Text:In the presence of a small amount of bromine, cyclohexene undergoes the following light-promoted reaction:
Br
hv
+ trace Br2
+ НBr
cyclohexene
3-bromocyclohexene
Part A
Propose a mechanism for this reaction.
Draw all missing reactants and/or products in the appropriate boxes by placing atoms on the canvas and connecting them with bonds. Add charges where needed. Electron
flow arrows should start on the electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created. Include all free radicals
by rightclicking on an atom on the canvas and then using the Atom properties to select the monovalent radical.
H
:Br
+H
CI
Br
Br i
O do
A mechanism must contain at least two rectangles, one containing starting materials and the other products.
No credit lost. Try again.
1 0 +1•
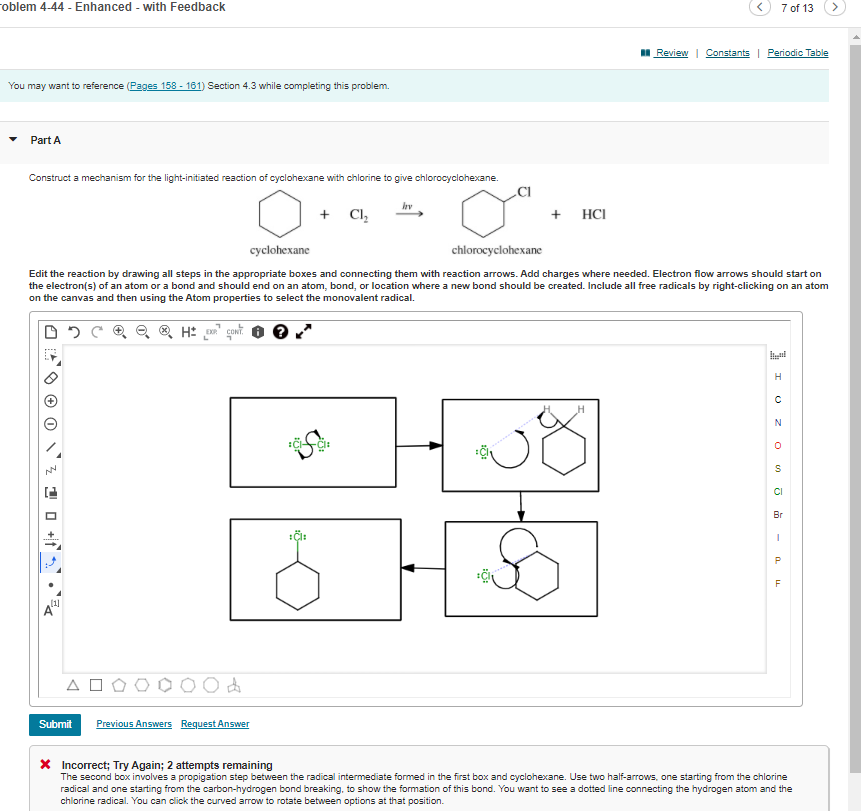
Transcribed Image Text:roblem 4-44 - Enhanced - with Feedback
7 of 13
I Review | Constants
Periodic Table
You may want to reference (Pages 158 - 161) Section 4.3 while completing this problem.
Part A
Construct a mechanism for the light-initiated reaction of cyclohexane with chlorine to give chlorocyclohexane.
hv
Cl,
+
HCI
cyclohexane
chlorocyclohexane
Edit the reaction by drawing all steps in the appropriate boxes and connecting them with reaction arrows. Add charges where needed. Electron flow arrows should start on
the electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created. Include all free radicals by right-clicking on an atom
on the canvas and then using the Atom properties to select the monovalent radical.
H: cont.
H
N
CI
Br
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 2 attempts remaining
The second box involves a propigation step between the radical intermediate formed in the first box and cyclohexane. Use two half-arrows, one starting from the chlorine
radical and one starting from the carbon-hydrogen bond breaking, to show the formation of this bond. You want to see a dotted line connecting the hydrogen atom and the
chlorine radical. You can click the curved arrow to rotate between options at that position.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

