In thermodynamic of the dissolution of Borax lab, the student's graph is below. Calculate AS? (R= 8.314 x 10-3 kJ/mole K) LN of Ksp vs 1/Temperature in Kelvin LN Ksp 0 0.0031 0.0032 0.0033 0.0034 0.0035 0.0036 0.0037 -2 -4 -6 y=-9104.5x + 26.39 R2 = 0.9713 Temperature in K-1 Series1 .........Linear (Series1) OA) 0.008075 kJ/mol K B) -75.69 kJ/mol K OC) -0.008075 kJ/mol K OD) -0.2194 kJ/mol K OE) 75.69 kJ/mol K OF) 0.2194 kJ/mol K
In thermodynamic of the dissolution of Borax lab, the student's graph is below. Calculate AS? (R= 8.314 x 10-3 kJ/mole K) LN of Ksp vs 1/Temperature in Kelvin LN Ksp 0 0.0031 0.0032 0.0033 0.0034 0.0035 0.0036 0.0037 -2 -4 -6 y=-9104.5x + 26.39 R2 = 0.9713 Temperature in K-1 Series1 .........Linear (Series1) OA) 0.008075 kJ/mol K B) -75.69 kJ/mol K OC) -0.008075 kJ/mol K OD) -0.2194 kJ/mol K OE) 75.69 kJ/mol K OF) 0.2194 kJ/mol K
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter13: Electrochemistry
Section: Chapter Questions
Problem 13.90PAE: A chemical engineering student is studying the effect of pH on the corrosion of iron. Ellie...
Related questions
Question
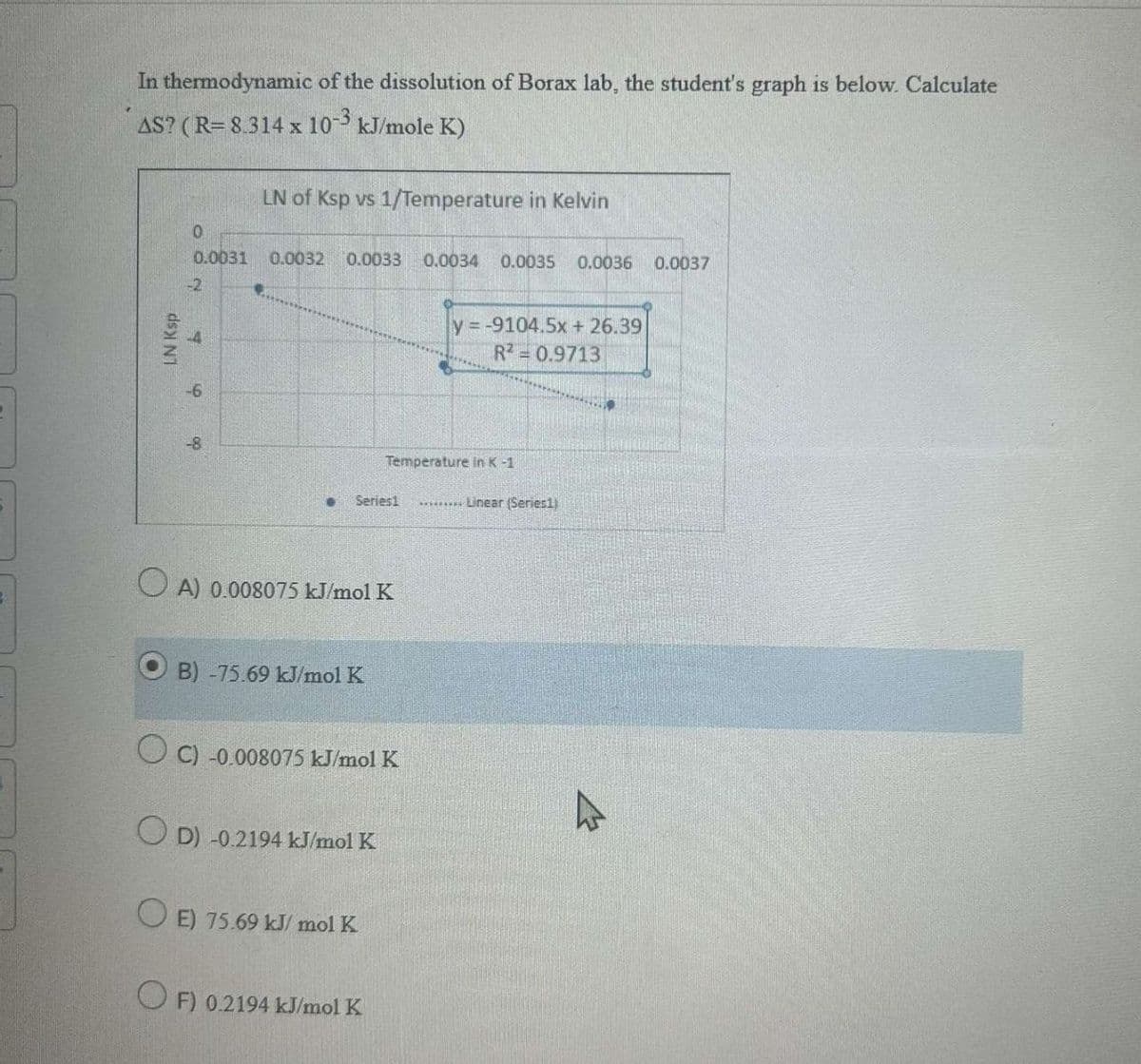
Transcribed Image Text:In thermodynamic of the dissolution of Borax lab, the student's graph is below. Calculate
AS? (R= 8.314 x 10-3 kJ/mole K)
LN of Ksp vs 1/Temperature in Kelvin
LN Ksp
0
0.0031 0.0032 0.0033
0.0034
0.0035 0.0036 0.0037
-2
-4
-6
y=-9104.5x + 26.39
R2 = 0.9713
Temperature in K-1
Series1
.........Linear (Series1)
OA) 0.008075 kJ/mol K
B) -75.69 kJ/mol K
OC) -0.008075 kJ/mol K
OD) -0.2194 kJ/mol K
OE) 75.69 kJ/mol K
OF) 0.2194 kJ/mol K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning