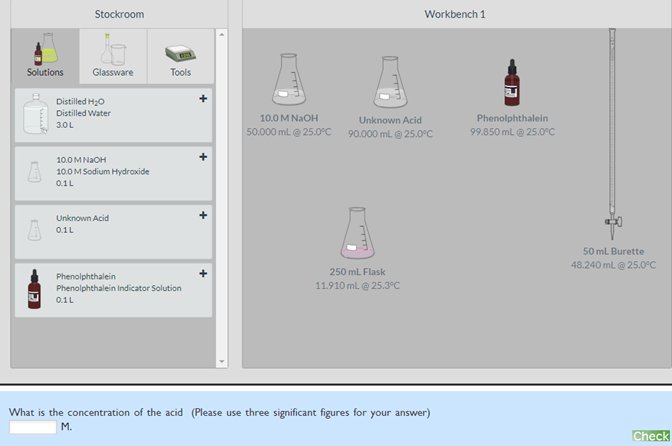
In this activity, you will use the virtual lab to determine the concentration of a strong monoprotic acid. To do this, you can perform a titration using NaOH and phenolphthalein found in the virtual lab. (Note: The concentration of the acid is between 0.025M and 2.5M so you will need to dilute the NaOH solution so that the volume to reach the endpoint is between 10 and 50 mL). 1. Volume (mL) of the unknown acid solution with the indicator in the flask: ________ (Write your answer in 2 decimal places without the unit). 2. Volume (mL) of the base used to complete the titration:____________ (Write your answer in 2 decimal places without the unit). 3. Concentration (Molarity) of the unknown acid:_________ (Write your answer in 2 decimal places without the unit).
In this activity, you will use the virtual lab to determine the concentration of a strong monoprotic acid. To do this, you can perform a titration using NaOH and phenolphthalein found in the virtual lab. (Note: The concentration of the acid is between 0.025M and 2.5M so you will need to dilute the NaOH solution so that the volume to reach the endpoint is between 10 and 50 mL).
1. Volume (mL) of the unknown acid solution with the indicator in the flask: ________ (Write your answer in 2 decimal places without the unit).
2. Volume (mL) of the base used to complete the titration:____________ (Write your answer in 2 decimal places without the unit).
3. Concentration (Molarity) of the unknown acid:_________ (Write your answer in 2 decimal places without the unit).
Step by step
Solved in 6 steps with 1 images









