In XCI, which crystallizes in CSCI structure, the sum of the anionic and cationic radii is found to be 318 pm. The density of the compound is 7.799 g cm. Calculate the mass of the cation as g mol. Do not write units, only enter numerical values. Use two digits at most for decimal numbers, use dot (.) as decimal separator (i.e. two-point-fifty-one should be entered as 2.51). Do not use comma () as decimal separator.
In XCI, which crystallizes in CSCI structure, the sum of the anionic and cationic radii is found to be 318 pm. The density of the compound is 7.799 g cm. Calculate the mass of the cation as g mol. Do not write units, only enter numerical values. Use two digits at most for decimal numbers, use dot (.) as decimal separator (i.e. two-point-fifty-one should be entered as 2.51). Do not use comma () as decimal separator.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter21: Structure And Bonding In Solids
Section: Chapter Questions
Problem 39P
Related questions
Question
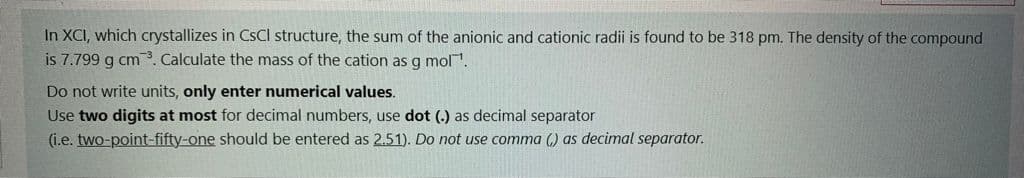
Transcribed Image Text:In XCI, which crystallizes in CSCI structure, the sum of the anionic and cationic radii is found to be 318 pm. The density of the compound
is 7.799 g cm. Calculate the mass of the cation as g mol.
Do not write units, only enter numerical values.
Use two digits at most for decimal numbers, use dot (.) as decimal separator
(i.e. two-point-fifty-one should be entered as 2.51). Do not use comma (,) as decimal separator.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning