Incorrect Your answer is wrong. In addition to checking your math, check that you used the right data and DID NOT round any intermediate calculations. A solution is prepared at 25 °c that is initially 0.92ì in ammonia (NH3), a weak base with K₁ = 1.8×10¯5, and 1.5M in ammonium bromide (NH4Br). Calculate the pH of the solution. Round your answer to 2 decimal places. pH = 10.89 Try one last time
Incorrect Your answer is wrong. In addition to checking your math, check that you used the right data and DID NOT round any intermediate calculations. A solution is prepared at 25 °c that is initially 0.92ì in ammonia (NH3), a weak base with K₁ = 1.8×10¯5, and 1.5M in ammonium bromide (NH4Br). Calculate the pH of the solution. Round your answer to 2 decimal places. pH = 10.89 Try one last time
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter14: Acids And Bases
Section: Chapter Questions
Problem 32QRT
Related questions
Question
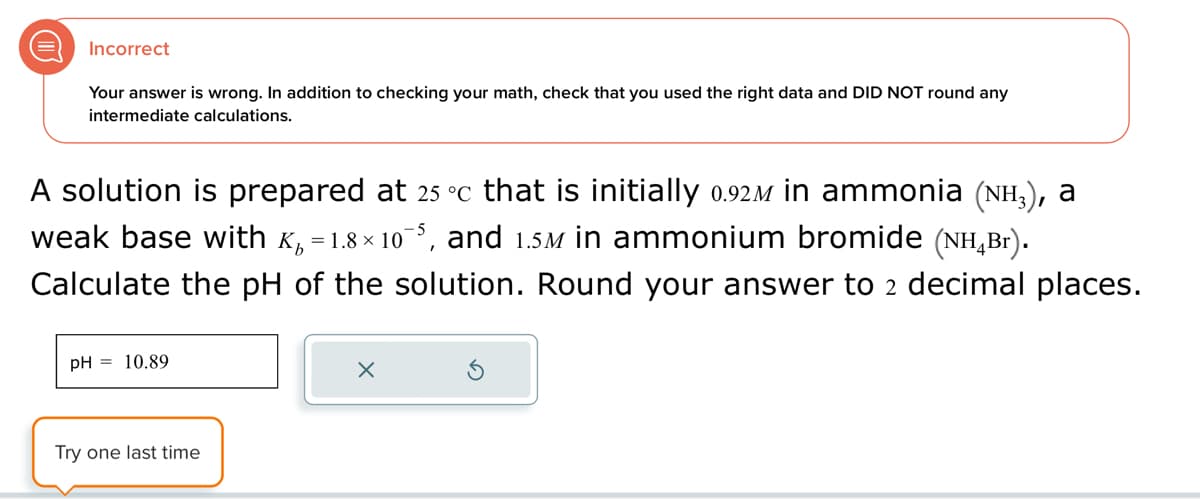
Transcribed Image Text:Incorrect
Your answer is wrong. In addition to checking your math, check that you used the right data and DID NOT round any
intermediate calculations.
A solution is prepared at 25 °c that is initially 0.92ì in ammonia (NH3), a
weak base with K₁ = 1.8×10¯5, and 1.5M in ammonium bromide (NH4Br).
Calculate the pH of the solution. Round your answer to 2 decimal places.
pH = 10.89
Try one last time
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning



Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning



Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning