ind the boiling point of a solution that is made from mixing 6.50 g of vanilin (MW= 153 g/mol) in 60.0g of ethanol (Use the table). BP of pure ethanol is 78.5 degrees Celsius.
Q: 2. Write down the chemical reaction for the heating of the hydrated form of copper II sulfate. The…
A: Mass of hydrated copper sulphate = 4.1g Mass of anhydrated copper sulphate = 2.6g Mass of water =…
Q: a. One liquid has a temperature of 140°F and another liquid has a temperature of 60°C. Are the…
A: A measurement can be converted to two units by the conversion factor. The temperature in 0F can be…
Q: Naproxen (C14H1403) is a commercially important anti-inflammatory agent that can be isolated from…
A: " Since you have posted a question with multiple sub-parts , we will solve first three sub-parts for…
Q: Naproxen (C14H1403) is a commercially important anti-inflammatory agent that can be isolated from…
A:
Q: The normal boiling point of water is Group of answer choices none of these 32°F 0°F 373 K 273 K
A:
Q: Water's heat of fusion is 80. cal/g and its specific heat is 1.0 * (cal)/(gC) Some velomobile seats…
A: Given : Mass of ice i.e frozen water = 1500 g Initial temperature of ice = 0.0 oC Final temperature…
Q: What are the components of the apparatus required for determining the boiling point of a liquid?. b)…
A: If only small amount of liquid material available for the boiling point determination, then a micro…
Q: А) 280 K B) The freezing point of water c) 5°C D The freezing point of water and 280 K (E) 30 °F
A:
Q: In a coffee-cup calorimeter, 29.8 g sample of solid KCl is dissolved in 100.0 g of water at 15.0 °C.…
A: Heat of solution :- It is measured by the change of enthalpy during formation of solution. This can…
Q: Suppose it is winter and the system is filled with methanol. The density of methanol is 0.80 g/cm3.…
A:
Q: A 31.5 g wafer of pure gold initially at 69.8°C is submerged into 63.4 g of water at 27.8 °C in an…
A: q = c*m*∆T q = heat c = specific heat capacity m is the mass of substance ∆T is the change in…
Q: A 25.0-g glass tumbler contains 200 mL of water at 24.0°C. If two 15.0-g ice cubes, each at a…
A:
Q: For NO2(g)NO2(g) find the value of ΔH∘fΔHf∘. Express your answer using four significant figures.…
A:
Q: Compare the effect of drinking 250-ml of ice water with the cooling effect of sweating out 250-ml of…
A: We sweat when our environment is hot or when we exercise. Sweating is a life-saving strategy that…
Q: Šuppose you have an ionic compound that has a solubility of 35g of MX per 100g water at 20°C. How…
A: Solubility is the amount of solute added in a given amount of solvent to attain saturation.
Q: Intensive properties independent of the amount of the substance in the system Select one: True False
A: Intensive property : A property which independent on the amount of matter present in a system is…
Q: An eager scientist on a hot summer day needs to determine how much ice to buy. She has a cooler…
A: The Initial temperature of soda = 86.5 ◦F The Final temperature of soda = 40.8 ◦F Conversion of…
Q: Which of the following mixtures can be best separated by filtration? a. ethanol in water b.…
A: To solve this problem we have to know about filtration and evaporation .
Q: A chemist wanted to obtain pure water from an impure sample using simple distillation. The…
A:
Q: An eager scientist on a hot summer day needs to determine how much ice to buy. She has a cooler…
A: Specific heat capacity is the amount of heat that is required to rise a temperature of 1 kg…
Q: A 15.00 g sample of a solid substance is placed in 100.0 g of water at 25°C, and all of the solid…
A:
Q: Given the change in the temperature from adding 3.00 grams of potassium chloride to 100.00 mL of…
A: We have to find enthalpy of solution
Q: An organic chemist measures the temperature T of a solution in a reaction flask. Here is the result.…
A:
Q: If 5.0125 g of calcium chloride (CaCl2) is dissolved in 100.0 mL of water, the following data are…
A: First find the mass of water. Find the heat energy as follows,
Q: 1. Convert 360 K to oC. 2. Room temperature is -20°C. Convert to K.
A: Kelvin and celsius are two units of temperature. 0 °C is equal to 273 Kelvin. And 0 Kelvin is…
Q: A student shakes up a plastic 2-L bottle of cafbonated beverage and then quickly opens the bottle-
A: ANS: EFFERVESCENCE CARBONATED BEVERAGES HAVE CO2,CO2 DISSOLVED IN UNDER PRESSURE WHEN BOTTLE…
Q: 1. A quart of boiling water will cause a more severe burn if it's spilled on you than just a drop of…
A:
Q: The ionic salt KBr is dissolved in water, much like the experiments given in the simulation. A mass…
A:
Q: 1. Which of the following accurately describes the conditions of an isolated system? a. Both energy…
A: The reaction is which heat is added is called as endothermic reaction. The reaction in which heat…
Q: Ethylene glycol, C2H6O2, is infinitely miscible (soluble) in water. It is a nonelectrolyte that is…
A:
Q: A chemistry student needs 90.0g of acetone for an experiment. She has available 420.g of a 44.1% w/w…
A: Solution stoichiometry involves the calculation of concentration of solutions in the given…
Q: What is the boiling point of the automobile radiator fluid prepared by mixing 1.04 L of ethylene…
A: Given : Volume of ethylene glycol = 1.04 L = 1040 mL…
Q: Equal amounts of three pure substances are mixed together. How many phases are present after mixing…
A: Matter has three main different forms- 1) Solid 2) liquid and 3) gas. But each solid and liquid can…
Q: D Other Bookmarks [References] Use the References to access important values if needed for this…
A:
Q: An organic chemist measures the temperature T of a solution in a reaction flask. Here is the result.…
A: Given that, T = 56℃ Change into K.
Q: How many grams of Na Cl would need to be added to 318.00 grams of water to lower the freezing point…
A: Given: Mass of water = 318.00 g = 0.318 Kg (Since 1 Kg = 1000 g)…
Q: Would the amount of heat absorbed by the dissolution as given below appear greater, lesser, or…
A: The answer for the first question is given below. Kindly repost the other questions as separate one
Q: The solubility of Fe(OH)2 in water at 25°C is measured to be 5.2x10^−4gL. Use this information to…
A: Solubility of Fe(OH)2 = 5.2 × 10-4 g/L Ksp for the Fe(OH)2 = ?
Q: An organic chemist measures the temperature T of a solution in a reaction flask. Here is the result.…
A: Temperature can be converted from °C to K using the conversion factor between these temperatures.
Q: A glucose solution contains 55.8 g of glucose (C6H12O6) in 465 g of water. 1. Calculate the…
A: Given data A glucose solution contains 55.8 g of glucose (C6H12O6) in 465 g of water. 1. the…
Q: Part A The heat of vaporization of water at 373 K is 40,7 kJ mol- Find q for the evaporation of 471…
A: Given the heat of vaporization of water at 373 K = 40.7 kJ.mol-1 Required conversion factor: 1 mol…
Q: For a 5.0% solution of CaCl2, calculate the: mass of CaCl2 in 360. grams of solution. amount of…
A:
Q: How many grams of sugar (C12H22O11) must dissolve in 10.0kg of water in order to melt the ice on a…
A: Ice melt at 0°C but if you want to melt the ice at -18°C. Then must be depression of freezing point.…
Q: If 400 cc of 15% (v/v) acetic acid is mixed with 800 cc of 35% (v/v) acetic acid, what are the total…
A:
Q: 1. Which of the following accurately describes the conditions of a closed system? a. Both energy and…
A: Please note: According to the guideline, in one session only three parts of a question can be…
Q: 11. The heat of solution ( Δ Hsoln) is the algebraic sum of the heat of mixing (Δ Hmix), the heat of…
A:
Q: Suppose that 4.24 grams of X (MW = 27.07) are dissolved in 602 mL of water, and the water…
A: The energy released or absorbed can be calculated by using following formula - Q = m C ∆T Here - Q…
Q: 3. A student goes to the cafeteria to get an iced tea. He puts 3 ice cubes (approximately 20.0 mL…
A: Given: volume of tea = 375 mL volume of ice = 20.0 mL Initial temperature of ice = -15.0 oC Initial…
Q: The temperature of 328.6 mL of Ne is changed from 274.49°C to 14.27K. What is the new volume of the…
A: Given,V1=328.6mL T1=274.49°C+273.15=547.64KV2=? T2=14.27KWe have…
Find the boiling point of a solution that is made from mixing 6.50 g of vanilin (MW= 153 g/mol) in 60.0g of ethanol (Use the table). BP of pure ethanol is 78.5 degrees Celsius.
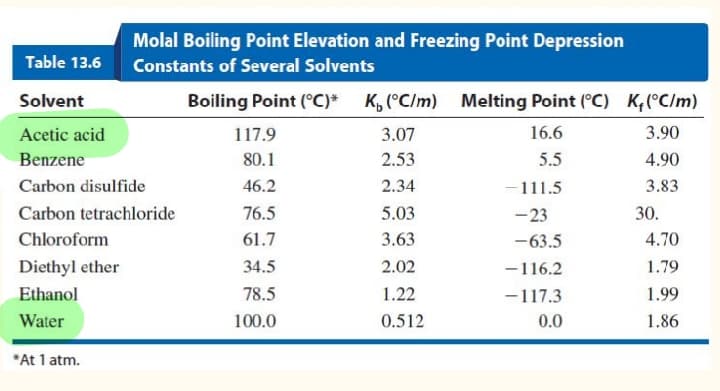
Step by step
Solved in 3 steps

- Boiling Point Elevation/Freezing Point DepressionT = m KWhere: T = T(solution) - T(pure solvent) * m = (# moles solute / Kg solvent) Kb = boiling point elevation constant. Kf = freezing point depression constant. Kb and Kf depend only on the SOLVENT. Below are some common values. Use these values for the calculations that follow. Solvent Formula Kb (°C / m) Kf (°C / m) Water H2O 0.512 -1.86 Ethanol CH3CH2OH 1.22 -1.99 Chloroform CHCl3 3.67 Benzene C6H6 2.53 -5.12 Diethyl ether CH3CH2OCH2CH3 2.02 note that ΔT as defined above will be a negative number for freezing point depression. Therefore, Kf must also be given as a negative number.Boiling Point Elevation/Freezing Point Depression T = m K where, for freezing point depression: T = T(pure solvent) - T(solution) and for boiling point elevation: T = T(solution) - T(pure solvent) m = (# moles solute / Kg solvent) Kb = boiling point elevation constant. Kf = freezing point depression constant. Kb and Kf depend only on the SOLVENT. Below are some common values. Use these values for the calculations that follow. Solvent Formula Kb(°C / m) Kf(°C / m) Water H2O 0.512 1.86 Ethanol CH3CH2OH 1.22 1.99 Chloroform CHCl3 3.67 Benzene C6H6 2.53 5.12 Diethyl ether CH3CH2OCH2CH3 2.02Bristol Community College Fall River, Massachusetts Experiment 6: Molar Mass of a Molecular Solid from Freezing Point-Depression Measurement Name: __________________________________ Date: _______________ Approved: ___________ DATA SHEET Mass of lauric acid (in Part II) Mass of benzoic acid (in Part II) Freezing temperature of pure lauric acid (from Part I) data from Video 2 of Part I Freezing temperature of solution (from Part II) data from Video referenced in Part II Freezing point depression, Tf ( = Tf, lauric acid – Tf, solution) Molality (m) of solution ( Eq. 1) Moles of benzoic acid ( Eq. 2) Experimental molar mass of benzoic acid (Eq. 3) Calculate the molar mass of benzoic acid, C6H5COOH. Percent error Summary Questions A student determines…
- Given that a mixture of nitric acid (bp 86 °C) and water forms a maximum-boiling-point azeotrope that boils at 120.7 °C with a composition of 67.4% nitric acid to 32.6% water. Construct an approximate boiling-point-phase diagram (with % composition on the x-axis and temperature on the y-axis) for this system. Include the boiling points of pure nitric acid, pure water, and the boiling point of the azeotrope on your diagram. Describe the behavior on distillation of a mixture that is 80% water and 20% nitric acid.1. What is the mole percent of methanol in your starting solution (composed of 1.5 mL and 6 mL isopropanol)? 2. Using the vapor-liquid equilibrium data graph (NOTE: You have to make this graph using the data provided) and your answer from question 1, answer the following: If your first distillate was 49 mole percent methanol, how many theoretical plates were in your distillation setup? Data Mole % of Methanol Temperature (°C) Vapor Liquid 66.22 95.35 90.10 67.94 89.10 79.00 70.22 80.00 66.05 72.67 68.50 52.20 74.78 57.00 40.80 77.06 42.85 29.30 78.94 29.00 19.50 81.00 13.20 8.10The experimental data in the table was collected during a freezing point depression study where BHT (butylated hydroxytoluene) was the solvent. Mass of BHT Mass of unknown Freezing point of pure BHT Freezing point of BHT and unknown solution Kf for BHT 7.709 g7.709 g 1.252 g1.252 g 74.17 ∘C74.17 ∘C 70.91 ∘C70.91 ∘C 6.83 ∘C/?6.83 ∘C/m Use this data to calculate the molar mass of the unknown solute. molar mass =
- What is the boiling point in Celsius of a solution containing 35.9g of h2O and 490.5g ethanol. molal depression ethanol = 1.22C Ethanol pure boiling = 78.4 Answer to 1 decimal placeA mixture of 20 mL of isoamyl acetate (MW=130.2 g/mol and density= 0.88 g/mL) and 20 mL of methyl benzoate (MW= 136.2 g/mol and density =1.09 g/mL) is distilled. Calculate the mole percent for each component. Use these mole percents and the figure below to answer the following questions.a. What is the initial boiling point of this mixture?b. What is the composition of the vapor in equilibrium with the liquid? Is this composition the same as the composition of the initial condensate from simple distillation?c. Instead of a simple distillation, you decide to use fractional distillation. Assuming two theoretical plates, what is the composition of the first fraction collected?Extra information: freezing point depression constant for the solvent (20.0 degrees Celcius kg/mol for cyclohexane)
- 1. Calculate the boiling point of a solution containing 12g glucoseC6H12O6 dissolved in 200g of water H2O. (0.5120C – Kb of H2O) 2. Calculate the normal freezing point of a 0.7439 mol aqueous solution of C12H22O11 that has a density of 1.35 g/ml. (C12H22O11 is a non-volatile non-dissociating solute.) The molal freezing point depression constant of water is 1.86OC kg/mole. 3. When 0.279 g of a molecular compound, benzoic acid, was dissolvedin 43.0 g of benzene, the freezing point of the solution was lowered to5.15 °C. What is the molecular weight of the benzoic acid? (Note: Kf forbenzene = 5.120C/m; Freezing point of benzene= 5.50C)In the lab instructions, it gives you the mass of the ice added to the calorimeter. Why is the mass of the ice important to obtain the molality? Hint: Think about what is present in the solution and how the molality gets computed. Instead of giving you the massed sample, the instructions now tell you to measure out 45g of ice. In your screenshot, show the balance with a beaker with ice massing ~45g. On in-person labs, you would grab an empty beaker, tare the mass of it, remove the beaker from the balance, add the ice, and then place it back on the balanceThe table below shows temperature/composition data collected for a mixture of methylbenzene (M) and octane (O) at 1 atm. Recall that x stands for the mole fraction in the liquid and y stands for the mole fraction in the vapor in equilibrium. The boiling points for methylbenzene (M) and octane (O) are 110.60C and 125.60C, respectively. Construct the phase diagram with Temperature vs. xM. What is the composition of the vapor in equilibrium with the liquid of composition (a) xM = 0.250 and (b) xO = 0.250. T (0C) 110.9 112.0 114.0 115.8 117.3 119.0 121.1 123.0 xM 0.908 0.795 0.615 0.527 0.408 0.300 0.203 0.097 yM 0.923 0.836 0.698 0.624 0.527 0.410 0.297 0.164

