Indole smells terrible in high concentrations but has a pleasant floral-like odor when highly diluted. Its structure is The molecule is planar, and the nitrogen is a very weak base, with Kp = 2 × 10-12. Explain how this information indicates that the indole molecule is aromatic.
Indole smells terrible in high concentrations but has a pleasant floral-like odor when highly diluted. Its structure is The molecule is planar, and the nitrogen is a very weak base, with Kp = 2 × 10-12. Explain how this information indicates that the indole molecule is aromatic.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter21: Benzene And The Concept Of Aromaticity
Section: Chapter Questions
Problem 21.16P: Which of the molecules and ions given in Problem 21.15 are aromatic according to the Hckel criteria?...
Related questions
Question
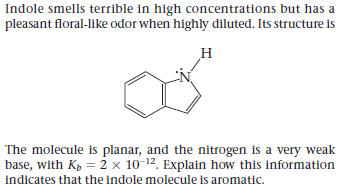
Transcribed Image Text:Indole smells terrible in high concentrations but has a
pleasant floral-like odor when highly diluted. Its structure is
The molecule is planar, and the nitrogen is a very weak
base, with Kp = 2 × 10-12. Explain how this information
indicates that the indole molecule is aromatic.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
