Industrially, nitric acid is produced by the Ostwald process, as represented by the following equations: 4NH3(g) + 50₂(g) → 4NO(g) + 6H₂0 (1) 2NO(g) + O₂(g) → 2NO₂(g) 2NO₂(g) + H₂O(1) → HNO3 (aq) + HNO₂ (aq) What mass of NH3 (in grams) must be used to produce 2.57 tons of HNO3 by the Ostwald process, assuming an 80.0 percent yield in each step (1 ton 2000 lb, 1 lb=453.6 g)?
Industrially, nitric acid is produced by the Ostwald process, as represented by the following equations: 4NH3(g) + 50₂(g) → 4NO(g) + 6H₂0 (1) 2NO(g) + O₂(g) → 2NO₂(g) 2NO₂(g) + H₂O(1) → HNO3 (aq) + HNO₂ (aq) What mass of NH3 (in grams) must be used to produce 2.57 tons of HNO3 by the Ostwald process, assuming an 80.0 percent yield in each step (1 ton 2000 lb, 1 lb=453.6 g)?
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter5: Gases
Section: Chapter Questions
Problem 5.89PAE: 89 A number of compounds containing the heavier noble gases, and especially xenon, have been...
Related questions
Question
100%
Pls pls pls help me solve the following question and make sure it's correct 10000% asap and explain, it's really important, pls and thanks
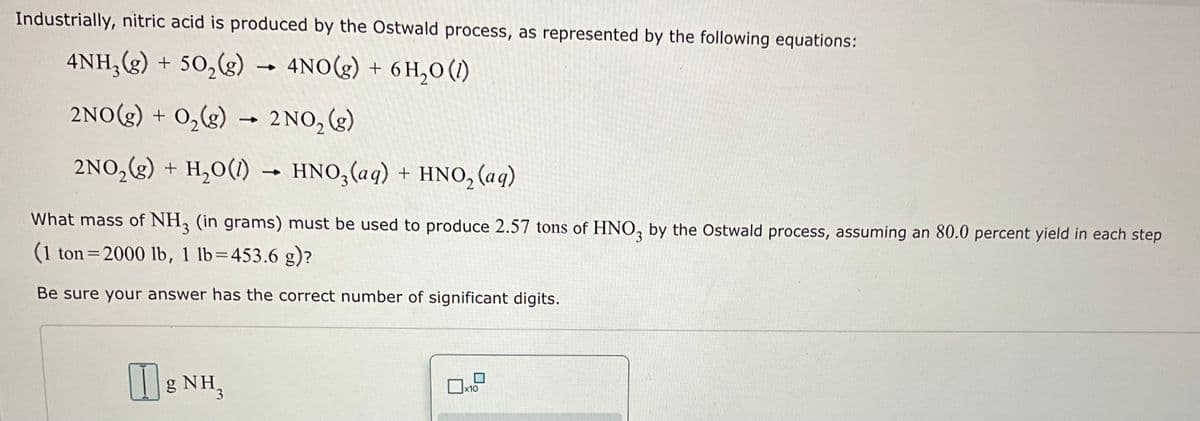
Transcribed Image Text:Industrially, nitric acid is produced by the Ostwald process, as represented by the following equations:
4NH₂(g) + 50₂(g) → 4NO(g) + 6H₂0 (1)
2NO(g) + O₂(g) → 2NO₂ (g)
2NO₂(g) + H₂O(1)→ HNO3 (aq) + HNO₂ (aq)
What mass of NH3 (in grams) must be used to produce 2.57 tons of HNO3 by the Ostwald process, assuming an 80.0 percent yield in each step
(1 ton=2000 lb, 1 lb=453.6 g)?
Be sure your answer has the correct number of significant digits.
gNH,
x10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step 1: Explaining the problem statement!
VIEWStep 2: Finding out the mass of nitric acid produced!
VIEWStep 3: Finding out moles of NO produced in first step!
VIEWStep 4: Finding out moles of NO2 produced in second step!
VIEWStep 5: Finding out moles of nitric acid produced in third step!
VIEWStep 6: Finding out the value of unknown y!
VIEWSolution
VIEWStep by step
Solved in 7 steps with 15 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning