Instruction: (a) Based on the information provided, determine the structures of compounds. (b) Assign all peaks in ¹H and C NMR spectra of compounds Compound 4: IR Spectrum (quid ) 4000 88888 dadadadadadadadadad % of base peak www 40 10 3000 13C NMR Spectrum (50.0 MHz, CDCI, solution) DEPT 80 proton decoupled 9 200 ¹H NMR Spectrum (200 MHz, CDCI, solution) 8 ww 2000 120 v (cm¹) m/e 160 160 7 1600 expansion 3.0 M 198/200 6 1200 200 240 120 Mass Spectrum 800 Resolves into two signals at higher field 2.5 C₂H₁1Br 280 Resolves into two signals at higher eld 5 solvent 80 ppm 0.0 1.0 3 wwwwwww absorbance 200 40 UV spectrum 5.30 mg/10 mis path length: 1.00 cm solvent: cyclohexane 250 2 expansions 2 (nm) 300 35.0 34.0 0 1 ppm 350 8 (ppm) TMS 0 8 (ppm)
Instruction: (a) Based on the information provided, determine the structures of compounds. (b) Assign all peaks in ¹H and C NMR spectra of compounds Compound 4: IR Spectrum (quid ) 4000 88888 dadadadadadadadadad % of base peak www 40 10 3000 13C NMR Spectrum (50.0 MHz, CDCI, solution) DEPT 80 proton decoupled 9 200 ¹H NMR Spectrum (200 MHz, CDCI, solution) 8 ww 2000 120 v (cm¹) m/e 160 160 7 1600 expansion 3.0 M 198/200 6 1200 200 240 120 Mass Spectrum 800 Resolves into two signals at higher field 2.5 C₂H₁1Br 280 Resolves into two signals at higher eld 5 solvent 80 ppm 0.0 1.0 3 wwwwwww absorbance 200 40 UV spectrum 5.30 mg/10 mis path length: 1.00 cm solvent: cyclohexane 250 2 expansions 2 (nm) 300 35.0 34.0 0 1 ppm 350 8 (ppm) TMS 0 8 (ppm)
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
ChapterL4: Proton (1h) Nmr Spectroscopy
Section: Chapter Questions
Problem 23CTQ
Related questions
Question
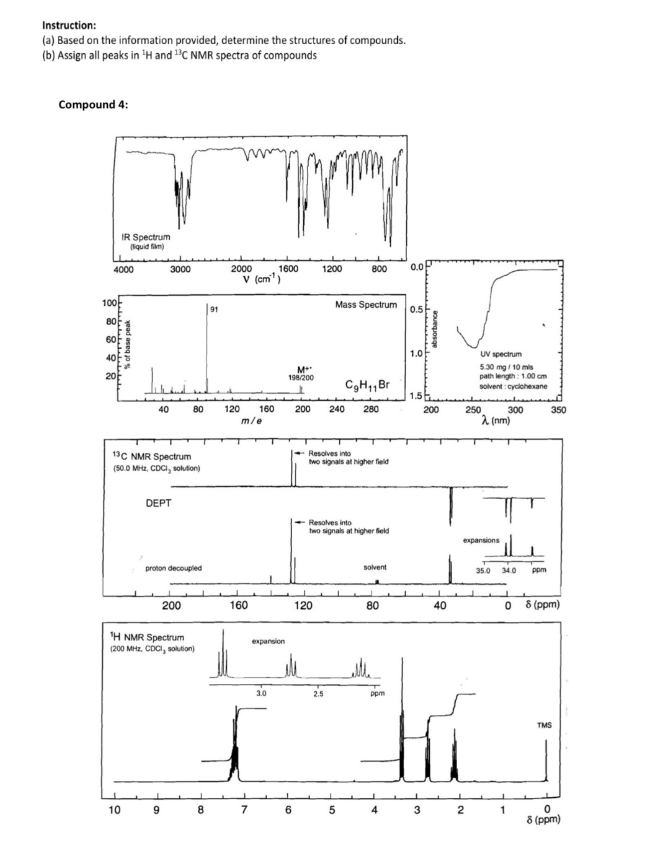
Transcribed Image Text:Instruction:
(a) Based on the information provided, determine the structures of compounds.
(b) Assign all peaks in ¹H and ¹³C NMR spectra of compounds
Compound 4:
4000
IR Spectrum
(liquid film)
100k
80
60
of base peak
% of
10
40
3000
Lind
13C NMR Spectrum
(50.0 MHz, CDCI, solution)
DEPT
proton decoupled
9
200
¹H NMR Spectrum
(200 MHz, CDCI, solution)
91
80 120 160
m/e
8
2000
v (cm¹)
160
7
1600
expansion
3.0
M**
198/200
6
200
1200
120
800
Mass Spectrum
2.5
Resolves into
two signals at higher field
CgH₁1 Br
240 280
Resolves into
two signals at higher field
10
solvent
80
ppm
0.0
0.5
1.0
1.5
3
absorbance
200
40
UV spectrum
5.30mg/10mis
path length: 1.00 cm
solvent: cyclohexane
250
2
expansions
2 (nm)
300
35.0 34.0
0
1
ppm
350
8 (ppm)
TMS
0
8 (ppm)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 10 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning
