Introduction Increasing use of industry (in particular, combustion processes) by humans is increasing the amount of carbon dioxide present in our atmosphere. One of the effects of this is increased solubility of carbon dioxide into the earth's waters, particularly the oceans. This has an effect on altering the pH of seawater. This should must include related chemical vocabulary, symbols and conventions throughout the answers. This should include relevant equations, equilibrium principles (including Ka) and pH principles. Calculations can be included. Q1: Describe the causes of increasing CO2 concentrations in the atmosphere Q2: Describe the chemical processes involved in CO, solubility into the oceans waters and the link between this and the ocean pH. Include appropriate chemical equations and equilibrium principles. This could include a description of the carbon- cycle mechanism in oceans. Q3: How increasing concentrations of CO2 affect the survivability of organisms which live in the sea (planktons, coral, shellfish..). Q4: An evaluation of the impact and consequences of ocean acidification on marine life (food chains and ecosystems). Q5: Recommend and justify ways in which ocean acidification could be slowed down. Link to chemical equations/equilibria.
Introduction Increasing use of industry (in particular, combustion processes) by humans is increasing the amount of carbon dioxide present in our atmosphere. One of the effects of this is increased solubility of carbon dioxide into the earth's waters, particularly the oceans. This has an effect on altering the pH of seawater. This should must include related chemical vocabulary, symbols and conventions throughout the answers. This should include relevant equations, equilibrium principles (including Ka) and pH principles. Calculations can be included. Q1: Describe the causes of increasing CO2 concentrations in the atmosphere Q2: Describe the chemical processes involved in CO, solubility into the oceans waters and the link between this and the ocean pH. Include appropriate chemical equations and equilibrium principles. This could include a description of the carbon- cycle mechanism in oceans. Q3: How increasing concentrations of CO2 affect the survivability of organisms which live in the sea (planktons, coral, shellfish..). Q4: An evaluation of the impact and consequences of ocean acidification on marine life (food chains and ecosystems). Q5: Recommend and justify ways in which ocean acidification could be slowed down. Link to chemical equations/equilibria.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 12.40PAE: Because carbonic acid undergoes a second ionization, the student in Exercise 12.39 is concerned that...
Related questions
Question
100%
this is my homework practice assignment that is very important and I really need help please give the answer for the 2 questions which I have highlighted with the green color I need a step by step explanation please please please help me.
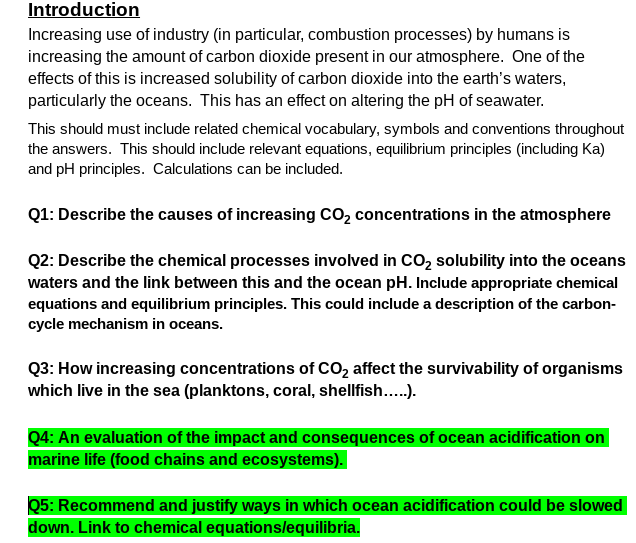
Transcribed Image Text:Introduction
Increasing use of industry (in particular, combustion processes) by humans is
increasing the amount of carbon dioxide present in our atmosphere. One of the
effects of this is increased solubility of carbon dioxide into the earth's waters,
particularly the oceans. This has an effect on altering the pH of seawater.
This should must include related chemical vocabulary, symbols and conventions throughout
the answers. This should include relevant equations, equilibrium principles (including Ka)
and pH principles. Calculations can be included.
Q1: Describe the causes of increasing CO2 concentrations in the atmosphere
Q2: Describe the chemical processes involved in Co, solubility into the oceans
waters and the link between this and the ocean pH. Include appropriate chemical
equations and equilibrium principles. This could include a description of the carbon-
cycle mechanism in oceans.
Q3: How increasing concentrations of CO2 affect the survivability of organisms
which live in the sea (planktons, coral, shellfish...).
Q4: An evaluation of the impact and consequences of ocean acidification on
marine life (food chains and ecosystems).
Q5: Recommend and justify ways in which ocean acidification could be slowed
down. Link to chemical equationslequilibria.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning