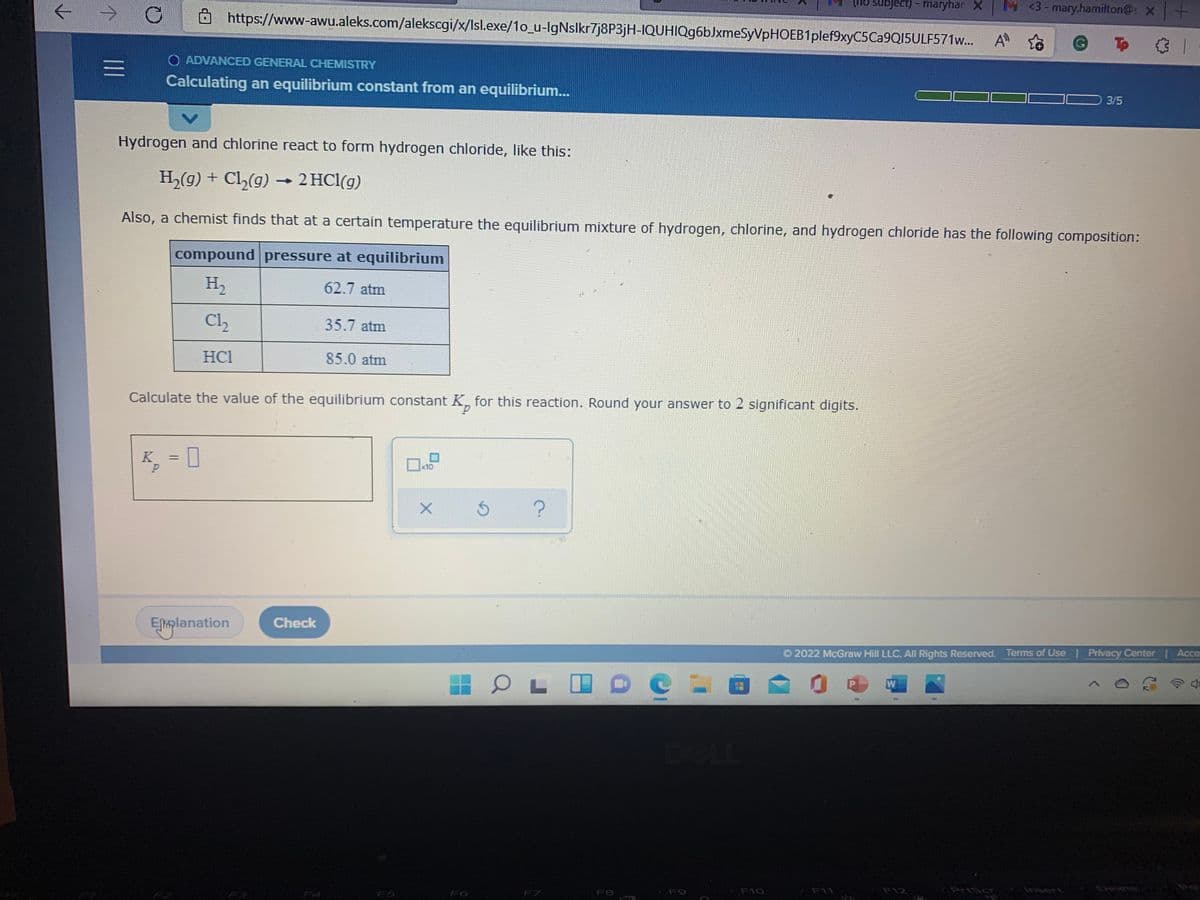
subject) - maryhar X M <3- mary.h Ô https://www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IQUHIQg6bJxmeSyVpHOEB1plef9xyC5Ca9QI5ULF571w.. A o O ADVANCED GENERAL CHEMISTRY Calculating an equilibrium constant from an equilibrium. Hydrogen and chlorine react to form hydrogen chloride, like this: H,(g) + Cl,(g) 2 HCl(g) Also, a chemist finds that at a certain temperature the equilibrium mixture of hydrogen, chlorine, and hydrogen chloride has the following com compound pressure at equilibrium H, 62.7 atm Cl, 35.7 atm HCI 85.0 atm Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits. K = | 1II
subject) - maryhar X M <3- mary.h Ô https://www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IQUHIQg6bJxmeSyVpHOEB1plef9xyC5Ca9QI5ULF571w.. A o O ADVANCED GENERAL CHEMISTRY Calculating an equilibrium constant from an equilibrium. Hydrogen and chlorine react to form hydrogen chloride, like this: H,(g) + Cl,(g) 2 HCl(g) Also, a chemist finds that at a certain temperature the equilibrium mixture of hydrogen, chlorine, and hydrogen chloride has the following com compound pressure at equilibrium H, 62.7 atm Cl, 35.7 atm HCI 85.0 atm Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits. K = | 1II
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.10QAP
Related questions
Question
Transcribed Image Text:->
https://www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IQUHIQg6bJxmeSyVpHOEB1plef9xyC5Ca9QI15ULF571w... A to
tho subject) - maryhan X
M <3- mary.hamilton@s X
To 3 1
O ADVANCED GENERAL CHEMISTRY
Calculating an equilibrium constant from an equilibrium..
3/5
Hydrogen and chlorine react to form hydrogen chloride, like this:
H,(g) + Cl,(g) → 2 HCl(g)
Also, a chemist finds that at a certain temperature the equilibrium mixture of hydrogen, chlorine, and hydrogen chloride has the following composition:
compound pressure at equilibrium
H2
62.7 atm
Cl
35.7 atm
HCl
85.0 atm
Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits.
d.
K
D
x10
Eſplanation
Check
O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Usel Privacy Center | Acce
P
W
F7
F8
F9
F10
F11
F12
PriScr
risert
Delete
FA
FS
F6
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

