Iodine-131 (131) is a radioactive isotope that is used in the treatment of cancer of the thyroid. The natural tendency of the thyroid to take up iodine creates a pathway for which radiation (B¯ and y) emitted from this unstable nucleus can be directed onto the cancerous tumor with very little collateral damage to surrounding healthy tissue. Another advantage of the isotope is its relatively short half-life of 8 days. Calculate the binding energy Eg of iodine-131. The mass of 131| is 130.906124 u. EB = MeV Ев Calculate the binding energy per nucleon Ев MeV A
Iodine-131 (131) is a radioactive isotope that is used in the treatment of cancer of the thyroid. The natural tendency of the thyroid to take up iodine creates a pathway for which radiation (B¯ and y) emitted from this unstable nucleus can be directed onto the cancerous tumor with very little collateral damage to surrounding healthy tissue. Another advantage of the isotope is its relatively short half-life of 8 days. Calculate the binding energy Eg of iodine-131. The mass of 131| is 130.906124 u. EB = MeV Ев Calculate the binding energy per nucleon Ев MeV A
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter18: Nuclear Chemistry
Section: Chapter Questions
Problem 79QRT
Related questions
Question
100%
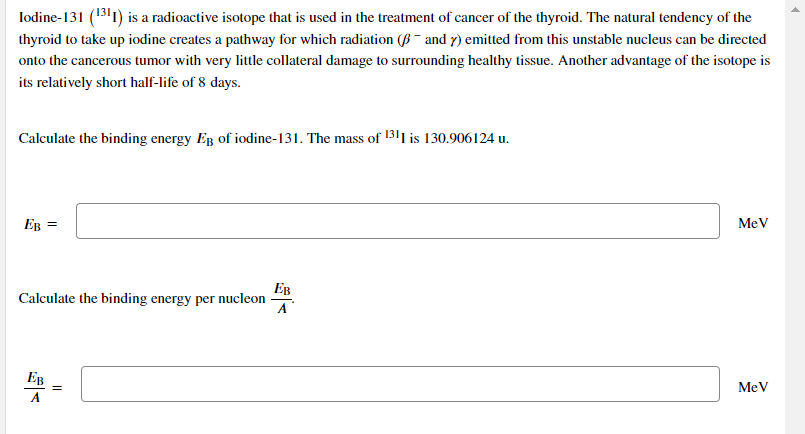
Transcribed Image Text:Iodine-131 (1311) is a radioactive isotope that is used in the treatment of cancer of the thyroid. The natural tendency of the
thyroid to take up iodine creates a pathway for which radiation (B ¯ and y) emitted from this unstable nucleus can be directed
onto the cancerous tumor with very little collateral damage to surrounding healthy tissue. Another advantage of the isotope is
its relatively short half-life of 8 days.
Calculate the binding energy Eg of iodine-131. The mass of 1311 is 130.906124 u.
EB =
MeV
Ев
Calculate the binding energy per nucleon
A
EB
MeV
A
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning