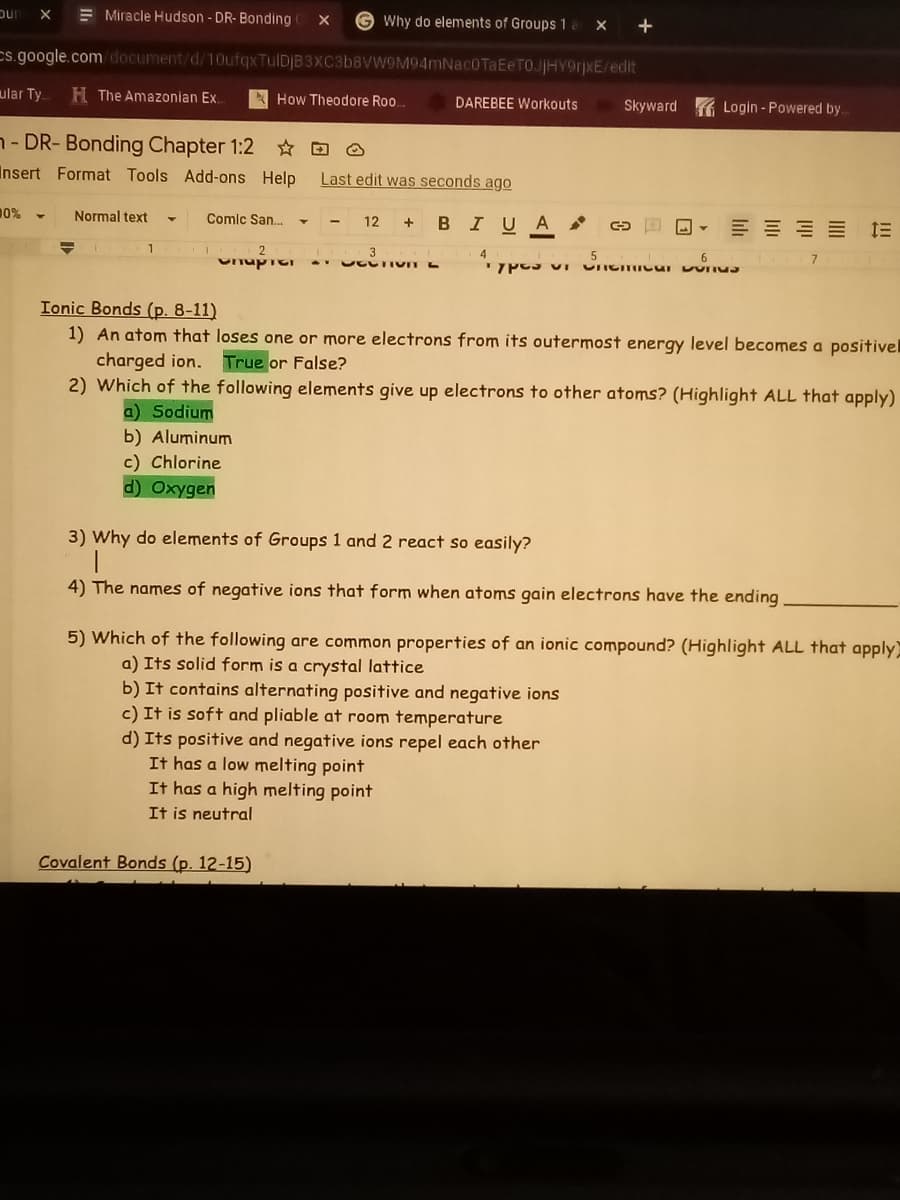
Ionic Bonds (p. 8-11) 1) An atom that loses one or more electrons from its outermost energy level becomes a positive charged ion. True or False? 2) Which of the following elements give up electrons to other atoms? (Highlight ALL that apply) a) Sodium b) Aluminum c) Chlorine d) Oxygen 3) Why do elements of Groups 1 and 2 react so easily? 4) The names of negative ions that form when atoms gain electrons have the ending 5) Which of the following are common properties of an ionic compound? (Highlight ALL that apply a) Its solid form is a crystal lattice b) It contains alternating positive and negative ions c) It is soft and pliable at room temperature d) Its positive and negative ions repel each other It has a low melting point It has a high melting point It is neutral
Ionic Bonds (p. 8-11) 1) An atom that loses one or more electrons from its outermost energy level becomes a positive charged ion. True or False? 2) Which of the following elements give up electrons to other atoms? (Highlight ALL that apply) a) Sodium b) Aluminum c) Chlorine d) Oxygen 3) Why do elements of Groups 1 and 2 react so easily? 4) The names of negative ions that form when atoms gain electrons have the ending 5) Which of the following are common properties of an ionic compound? (Highlight ALL that apply a) Its solid form is a crystal lattice b) It contains alternating positive and negative ions c) It is soft and pliable at room temperature d) Its positive and negative ions repel each other It has a low melting point It has a high melting point It is neutral
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter12: The Solid State
Section: Chapter Questions
Problem 60IL: Calculate the lattice energy of CaCl2 using a Born-Haber cycle and data from Appendices F and L and...
Related questions
Question
Transcribed Image Text:E Miracle Hudson - DR- Bonding
G Why do elements of Groups 1 a
cs.google.com document/d/10ufqxTulDjB3XC3b8VW9M94mNac0TaEeTOJjHY9rjxE/edit
ular Ty.
H The Amazonian Ex.
How Theodore Roo
DAREBEE Workouts
Skyward
G Login - Powered by.
7- DR- Bonding Chapter 1:2
Insert Format Tools Add-ons Help
Last edit was seconds ago
0%
Normal text
Comlc San.
в I U A >
12
E E = E
+
1
3
Ionic Bonds (p. 8-11)
1) An atom that loses one or more electrons from its outermost energy level becomes a positivel
charged ion.
2) Which of the following elements give up electrons to other atoms? (Highlight ALL that apply)
True or False?
a) Sodium
b) Aluminum
c) Chlorine
d) Oxygen
3) Why do elements of Groups 1 and 2 react so easily?
4) The names of negative ions that form when atoms gain electrons have the ending
5) Which of the following are common properties of an ionic compound? (Highlight ALL that apply)
a) Its solid form is a crystal lattice
b) It contains alternating positive and negative ions
c) It is soft and pliable at room temperature
d) Its positive and negative ions repel each other
It has a low melting point
It has a high melting point
It is neutral
Covalent Bonds (p. 12-15)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
