What does S do to become stable ? S will| and have a charge. a. lose 2 electrons b. lose 3 electrons C. gain 2 electrons d. gain 3 electrons e. lose 4 electrons f. gain 4 electrons g. +2 h. +3 i. -2 j. -3 k. +4 1. -4 Question 7 of 10 1 Points Give the complete electron configuration for the sulfur ion in problem 6. Do not use the noble gas abbreviation. The S ion has electrons 1s2 2s2 2p6 а. 10 b. 12 С. 14 d. 16 е. 18 f. 36 g. 38 i. 40 j. 3p6 h. 39 k. 3p4 I. 3s2 m. За10 n. 4d1 0. 4s2 р. 4p6 q. 5s2 r. 5s° S. 4dº t. 4d3 u. 5s1 v. 3p5
What does S do to become stable ? S will| and have a charge. a. lose 2 electrons b. lose 3 electrons C. gain 2 electrons d. gain 3 electrons e. lose 4 electrons f. gain 4 electrons g. +2 h. +3 i. -2 j. -3 k. +4 1. -4 Question 7 of 10 1 Points Give the complete electron configuration for the sulfur ion in problem 6. Do not use the noble gas abbreviation. The S ion has electrons 1s2 2s2 2p6 а. 10 b. 12 С. 14 d. 16 е. 18 f. 36 g. 38 i. 40 j. 3p6 h. 39 k. 3p4 I. 3s2 m. За10 n. 4d1 0. 4s2 р. 4p6 q. 5s2 r. 5s° S. 4dº t. 4d3 u. 5s1 v. 3p5
ChapterU2: Smells: Molecular Structure And Properties
Section: Chapter Questions
Problem 3STP
Related questions
Question
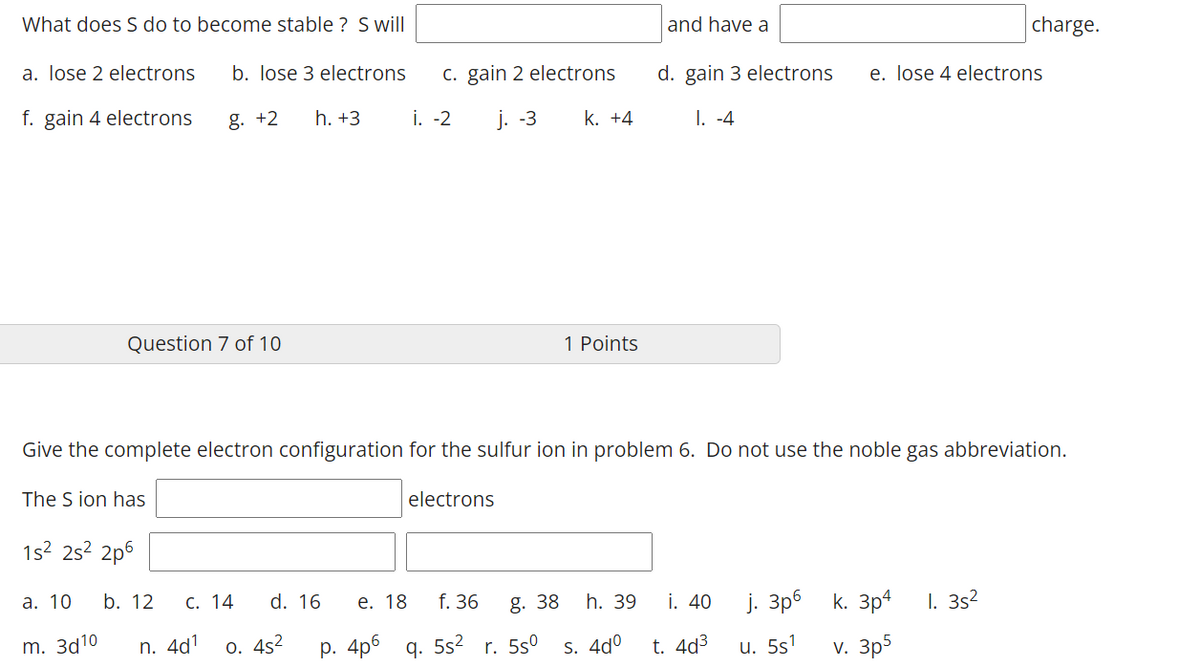
Transcribed Image Text:What does S do to become stable ? Swill
and have a
charge.
a. lose 2 electrons
b. lose 3 electrons
c. gain 2 electrons
d. gain 3 electrons
e. lose 4 electrons
f. gain 4 electrons
g. +2
h. +3
i. -2
j. -3
k. +4
I. -4
Question 7 of 10
1 Points
Give the complete electron configuration for the sulfur ion in problem 6. Do not use the noble gas abbreviation.
The S ion has
electrons
1s2 2s? 2p6
а. 10
d. 16
i. 40
j. 3p6 k. 3p4
I. 3s2
b. 12
С. 14
е. 18
f. 36
g. 38
h. 39
m. За10
n. 4d1
o. 4s2
р. 4p6 q. 552
r. 5s°
S. 4dº
t. 4d3
u. 5s1
v. 3p5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning



Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
