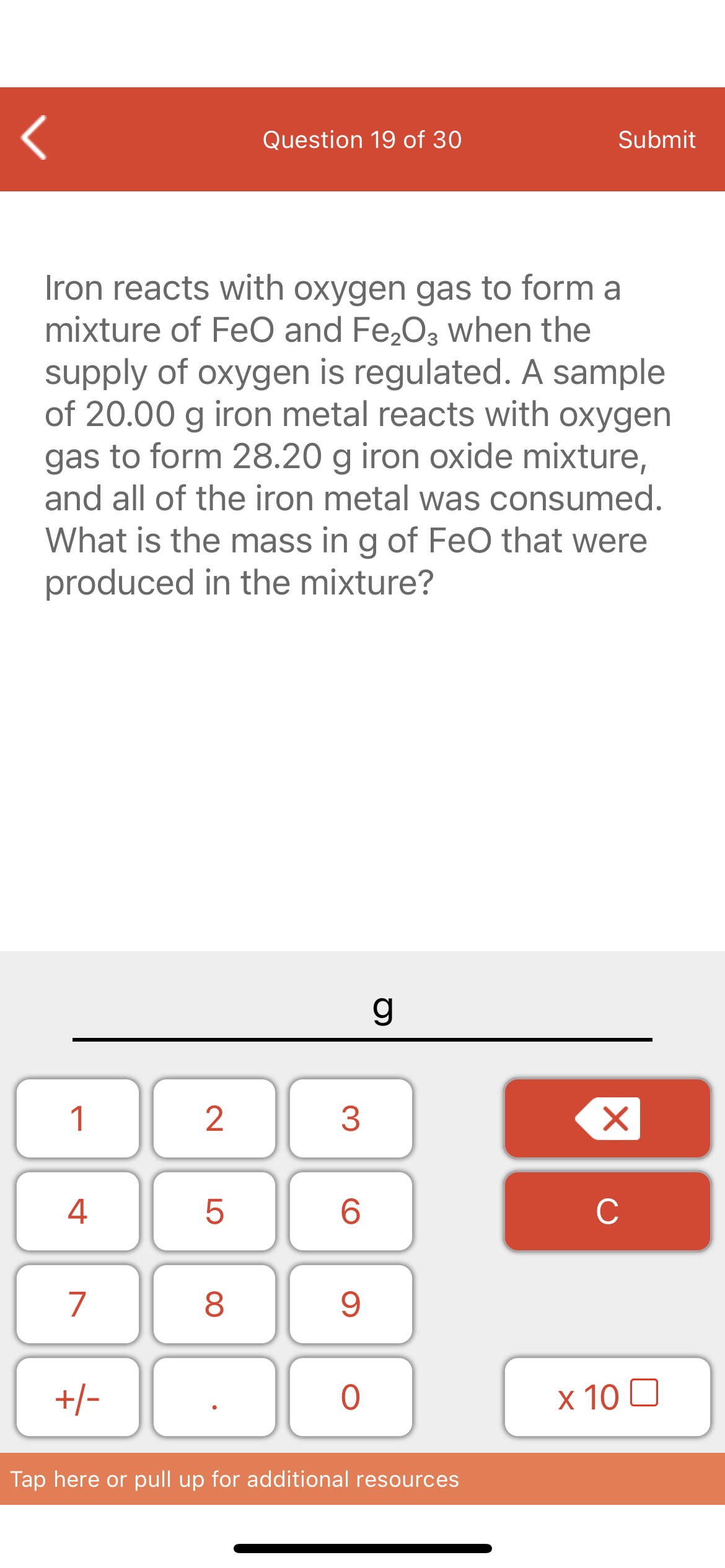
Iron reacts with oxygen gas to form a mixture of FeO and Fe,O3 when the supply of oxygen is regulated. A sample of 20.00 g iron metal reacts with oxygen gas to form 28.20 g iron oxide mixture, and all of the iron metal was consumed. What is the mass in g of FeO that were produced in the mixture?
Iron reacts with oxygen gas to form a mixture of FeO and Fe,O3 when the supply of oxygen is regulated. A sample of 20.00 g iron metal reacts with oxygen gas to form 28.20 g iron oxide mixture, and all of the iron metal was consumed. What is the mass in g of FeO that were produced in the mixture?
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 86AP: When small quantities of elemental hydrogen gas are needed for laboratory work, the hydrogen is...
Related questions
Question
Transcribed Image Text:Question 19 of 30
Submit
Iron reacts with oxygen gas to form a
mixture of FeO and Fe,O3 when the
supply of oxygen is regulated. A sample
of 20.00 g iron metal reacts with oxygen
gas to form 28.20 g iron oxide mixture,
and all of the iron metal was consumed.
What is the mass in g of FeO that were
produced in the mixture?
1
4
6.
C
7
+/-
х 10 0
Tap here or pull up for additional resources
LO
00
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning