It can be deduced from the rate equation of the reaction, CH-COCH, + l- CH,COCH;I + H + given by rate - K[CH COCH ][H'], that the reaction is 1 Second order 2 Zero order with respect to 2. 3 Unimolecular A. !! D
It can be deduced from the rate equation of the reaction, CH-COCH, + l- CH,COCH;I + H + given by rate - K[CH COCH ][H'], that the reaction is 1 Second order 2 Zero order with respect to 2. 3 Unimolecular A. !! D
Chapter5: Stereochemistry At Tetrahedral Centers
Section5.SE: Something Extra
Problem 58AP: One of the steps in fat metabolism is the hydration of crotonate to yield 3-hydroxybutyrate. This...
Related questions
Question
Show-all-working-explaining-detailly-each-step.
Answer should be typewritten using a computer keyboard
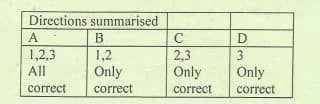
Transcribed Image Text:Directions summarised
A
B
1,2,3
1,2
2,3
Only
All
Only
Only
correct
correct
correct
correct
![It can be deduced from the rate equation of
35.
the reaction,
CH:COCH, + 1 CH,COCH;I + H +
given by rate = K[CH,COCH ][H'], that the
reaction is
1 Second order
2 Zero order with respect to I2.
3 Unimolecular
!3!
A](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F5a6d9c67-6f13-49d2-ac4d-2d996f90a88b%2F26d2ab4c-922e-45f9-94b8-8f582592661e%2Fo641maa_processed.jpeg&w=3840&q=75)
Transcribed Image Text:It can be deduced from the rate equation of
35.
the reaction,
CH:COCH, + 1 CH,COCH;I + H +
given by rate = K[CH,COCH ][H'], that the
reaction is
1 Second order
2 Zero order with respect to I2.
3 Unimolecular
!3!
A
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

