(iv) Arrange the hydroxides of Group II elements (Be to Ba) in decreasing order of solubility beginning with the most soluble. Explain the trend. (v) State two ways in which the chemistry of lithium or its compounds differ from those of other alkali elements.
(iv) Arrange the hydroxides of Group II elements (Be to Ba) in decreasing order of solubility beginning with the most soluble. Explain the trend. (v) State two ways in which the chemistry of lithium or its compounds differ from those of other alkali elements.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter12: Chemical Equilibrium
Section12.2: The Equilibrium Constant
Problem 12.2PSP: When carbon dioxide dissolves in water it reacts to produce carbonic acid, H2CO3(aq), which can...
Related questions
Question
Show all working explaining detailly each step.
Answer should be type written using a computer Keyboard!
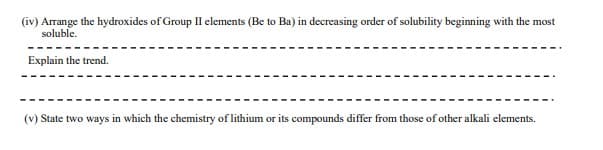
Transcribed Image Text:(iv) Arrange the hydroxides of Group II elements (Be to Ba) in decreasing order of solubility beginning with the most
soluble.
Explain the trend.
(v) State two ways in which the chemistry of lithium or its compounds differ from those of other alkali elements.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning