iv. Calculate the Gibbs free energy for the overall reaction. V. Write the standard cell notation for the overall reaction. vi. Calculate Keq for the spontaneous reaction.
iv. Calculate the Gibbs free energy for the overall reaction. V. Write the standard cell notation for the overall reaction. vi. Calculate Keq for the spontaneous reaction.
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter24: Coulometry
Section: Chapter Questions
Problem 24.4QAP: Halide ions can he deposited at a silver anode, the reaction being Ag(s) + X- AgX(s) +e- Suppose...
Related questions
Question
Need help with the last 3.
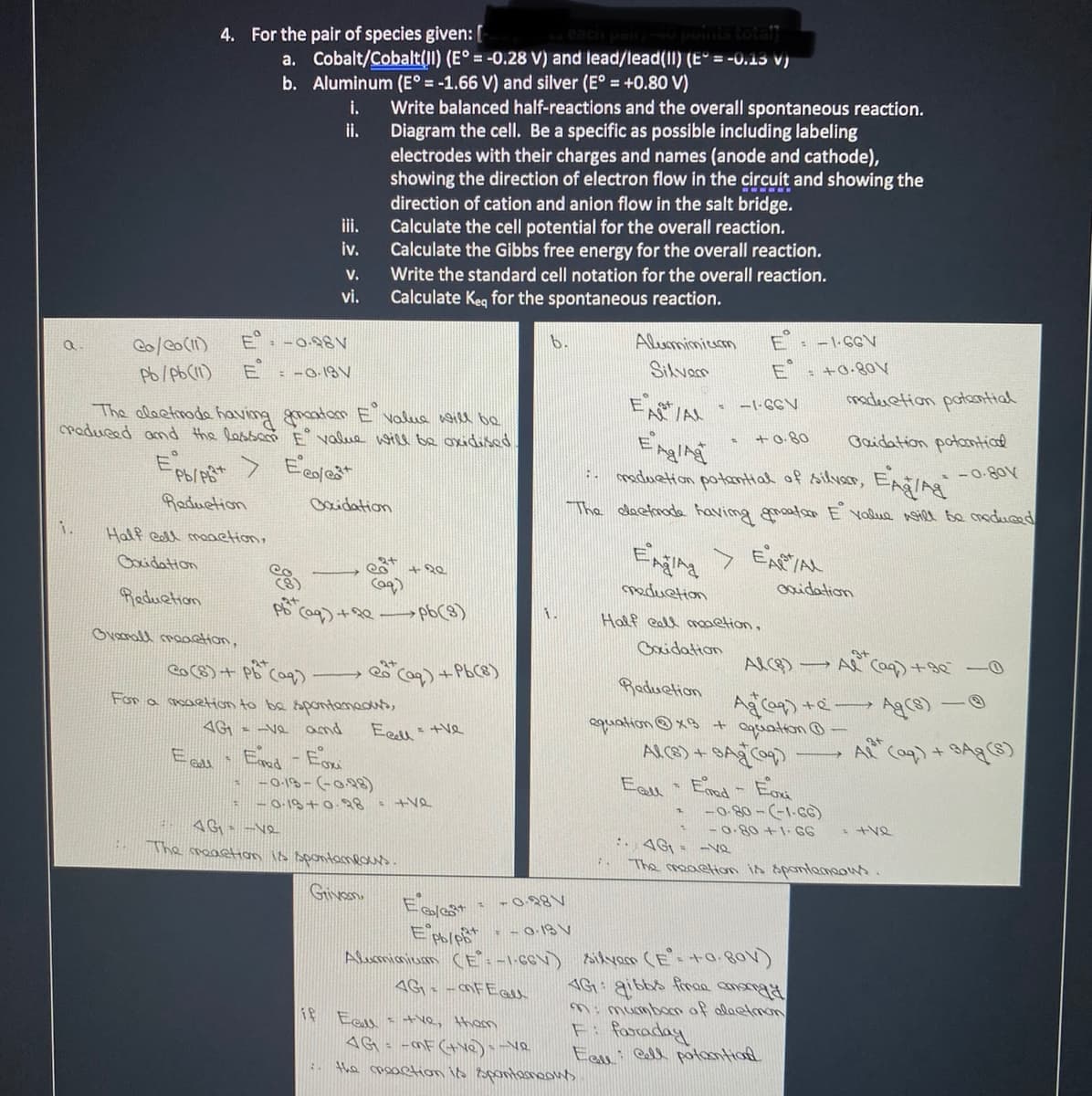
Transcribed Image Text:4. For the pair of species given: [
a. Cobalt/Cobalt(II) (E° = -0.28 V) and lead/lead(II) (E° = -0.13 V)
b. Aluminum (E° = -1.66 V) and silver (E° = +0.80 V)
Write balanced half-reactions and the overall spontaneous reaction.
ii.
i.
Diagram the cell. Be a specific as possible including labeling
electrodes with their charges and names (anode and cathode),
showing the direction of electron flow in the circuit and showing the
direction of cation and anion flow in the salt bridge.
iii.
Calculate the cell potential for the overall reaction.
iv.
Calculate the Gibbs free energy for the overall reaction.
V.
Write the standard cell notation for the overall reaction.
vi.
Calculate Keq for the spontaneous reaction.
E: -0.98V
b.
Alemimicrm
: - |-GGV
Pb/pb(11)
E : -0-18V
Silvarn
: +Q.80V
ー-CCN
maderetion potaontial
The alaetinode having gmoatom E valua will be
roduced and the lassam E value will be oxidised
+ 0.80
Gaidation potantial
maduetion potantial of silvar, EAgIng
- 0-80V
Raduetion
Baidation
The dactnada havimg groata E Yalue nil be maduced
Half eall maetion,
Ening > ExPAL
Cocidation
vorHompab
Half eall mmetion,
caidation
Reduetion
Oveenall maoction,
Gaidation
co(8) + PB
Ca)+PbC8)
→以 C)+ 0
Caq)
For a maetion to be spontenadus,
Raduetion
4G - -Ve and
Eell +ve
aquation©xB + aguation -
Eall
A Co)+ BAg)
-018-(-0,98)
-0.19 +0.28
Eall
(29-1-)- 080ー
-0.80 +1- GG
4G- -ve
The meactan ib spontamlous
:. 4G = -ve
Givan
- -O.18 V
Alusmigium CE.-1-6EV) sikyem (E-+a 8aV)
4G - -MFEall
AG: gibbs firoa an
Mi mumbon of alaetmom
Fi faraday
Eau Call potantiad
e Eal s tve, tham
4G - -mF (tve) e
the moaetion io Bpontamaws
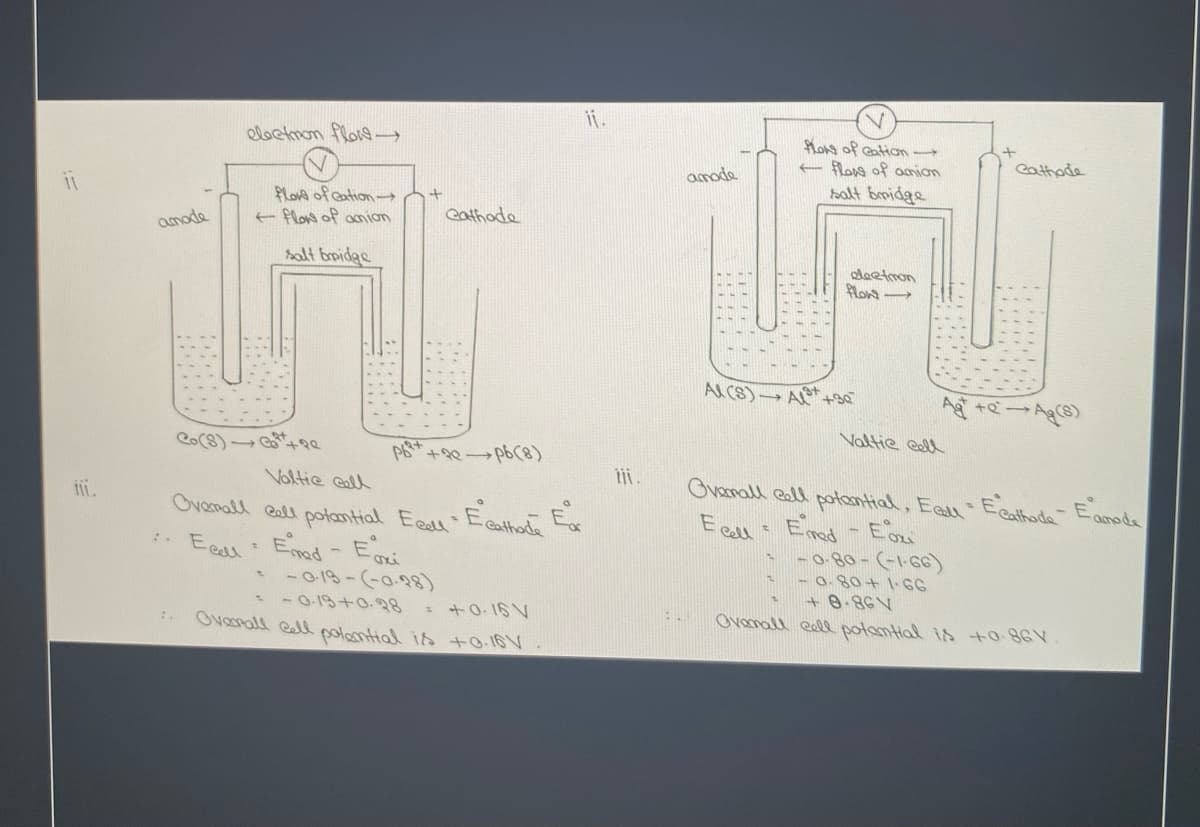
Transcribed Image Text:i.
Kos of ation
E flong of amion
halt bmidge
eloctmon flos
eathade
amade
love of Gation
e flow of amion
eathade
amode
halt bridgs
oleetmon
ACS) A +3e
Ag +eAg
Valtie eell
(8) +9e
Ovanall eall potontial, Eell Ecathode- Eamode
E call : Emad - Ezi
-0-80-(-1-66)
Voltie call
ii.
Ovamall esll potantial Eaal Ecathent Ex
: Eeal Emod - Eri
-013-(-0.98)
- - O-19+0.28
Ovanall ell poantial is tO-16V
+ @-86V
Ovamall ell potamtial is +0.86V
*+0. 16 V
:.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole