Chapter1: Matter, Measurements, And Calculations
Section: Chapter Questions
Problem 1.116E
Related questions
Question
Please refer to the molecule shown below when answering questions:
Based on relative trends in the periodic table, which function group is more basic, II or
VIII?
(i) Based on relative trends in the periodic table, which function group is more acidic, II or
VII?
(j) Because of ring strain, this functional group is likely to undergo ring-opening upon
reaction with HCl.
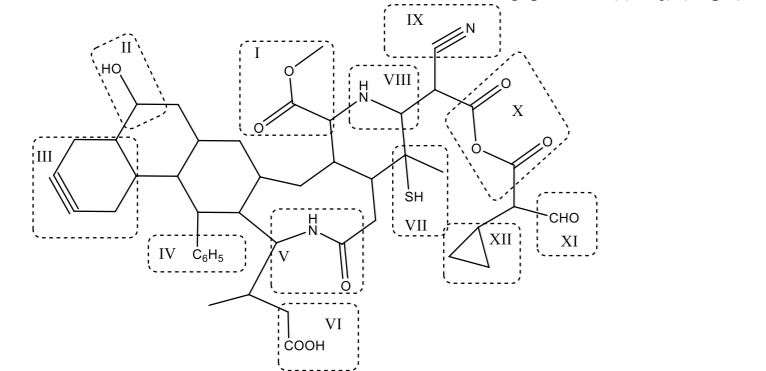
Transcribed Image Text:IX
II
HO
VIII
X
SH
H
VII
CHO
IV C3H5
XII
XI
VI
čOOH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

