Given that HO(CH2)2COOH has a pKa = 4.5 and HO(CH2)2 NH3 has a pKa = 9.5, answer the following: %3D %3D What pH would you make the water layer in order to cause both compounds to dissolve in it? Water should have pH 6.5 - 7.5. Water should have pH 4.5 - 9.5. Water should have pH 4.5 - 7.5. Water should have pH 6.5 - 9.5. What pH would you make the water layer in order to cause the acid to dissolve in the water layer and the amine to dissolve in the ether layer? Express your answer using one decimal place. ΑΣφ ? pH > What pH would you make the water layer in order to cause the acid to dissolve in the ether layer and the amine to dissolve in the water layer? Express vour answer using one
Given that HO(CH2)2COOH has a pKa = 4.5 and HO(CH2)2 NH3 has a pKa = 9.5, answer the following: %3D %3D What pH would you make the water layer in order to cause both compounds to dissolve in it? Water should have pH 6.5 - 7.5. Water should have pH 4.5 - 9.5. Water should have pH 4.5 - 7.5. Water should have pH 6.5 - 9.5. What pH would you make the water layer in order to cause the acid to dissolve in the water layer and the amine to dissolve in the ether layer? Express your answer using one decimal place. ΑΣφ ? pH > What pH would you make the water layer in order to cause the acid to dissolve in the ether layer and the amine to dissolve in the water layer? Express vour answer using one
Chapter20: Carboxylic Acids And Nitriles
Section20.4: Substituent Effects On Acidity
Problem 7P: Dicarboxylic acids have two dissociation constants, one for the initial dissociation into a...
Related questions
Question
Please answer all 3
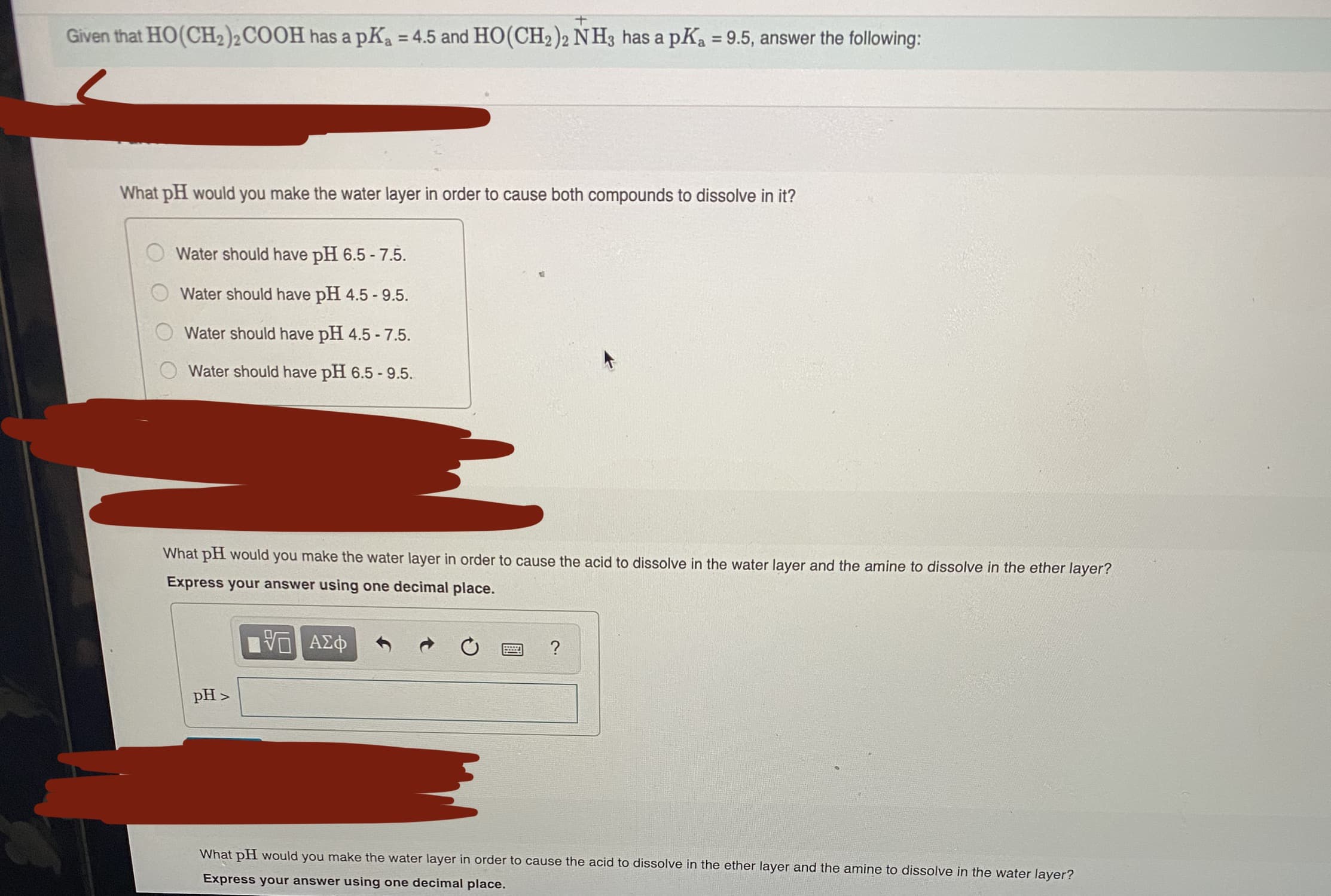
Transcribed Image Text:Given that HO(CH2)2COOH has a pKa = 4.5 and HO(CH2)2 NH3 has a pKa = 9.5, answer the following:
%3D
%3D
What pH would you make the water layer in order to cause both compounds to dissolve in it?
Water should have pH 6.5 - 7.5.
Water should have pH 4.5 - 9.5.
Water should have pH 4.5 - 7.5.
Water should have pH 6.5 - 9.5.
What pH would you make the water layer in order to cause the acid to dissolve in the water layer and the amine to dissolve in the ether layer?
Express your answer using one decimal place.
ΑΣφ
?
pH >
What pH would you make the water layer in order to cause the acid to dissolve in the ether layer and the amine to dissolve in the water layer?
Express vour answer using one
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning



Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning