KI: 0.250M (NH4)₂08 0.250M Nav 50g: 0.200 M KNOS: 0.250M 2- S₂09² (oop +2 I (aq) → I₂ (s) +250 ₂² Ia(s) + 2 S₂03² (aq)- 21- (aq) + 5406² Moles of Time (Seconds) $₂0,2- Run! Run 2 Run 3 O 0 0 0 0.0002 130 65 9.8 0.0004 260 140 206 0.0006 398 200 317 0.0008 547 288 432 0.001 703 362 559 Rate of Reaction of Thiosulfate for Each Run 2- Run Rate of SD (L molts) 1.43 x 10-6 2 2.75 x 10-6 3 1.79 x 10-6 Calculate the rate of reaction of 5₂082- three runs Use 50,0 ML Fill in the table below.. Rate of Reaction Peroxydisulfate for Each Run Run Rate of 5₂08² (MS+) 1 2 3 in Mst for the as the volume of the reaction mixtu
KI: 0.250M (NH4)₂08 0.250M Nav 50g: 0.200 M KNOS: 0.250M 2- S₂09² (oop +2 I (aq) → I₂ (s) +250 ₂² Ia(s) + 2 S₂03² (aq)- 21- (aq) + 5406² Moles of Time (Seconds) $₂0,2- Run! Run 2 Run 3 O 0 0 0 0.0002 130 65 9.8 0.0004 260 140 206 0.0006 398 200 317 0.0008 547 288 432 0.001 703 362 559 Rate of Reaction of Thiosulfate for Each Run 2- Run Rate of SD (L molts) 1.43 x 10-6 2 2.75 x 10-6 3 1.79 x 10-6 Calculate the rate of reaction of 5₂082- three runs Use 50,0 ML Fill in the table below.. Rate of Reaction Peroxydisulfate for Each Run Run Rate of 5₂08² (MS+) 1 2 3 in Mst for the as the volume of the reaction mixtu
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter21: The Chemistry Of The Main Group Elements
Section: Chapter Questions
Problem 120IL
Related questions
Question
Chemistry
Could you help me fill the rate of reaction peroxudisulfate table highlighted in the image below? Please write how to calculate and get to the answers.
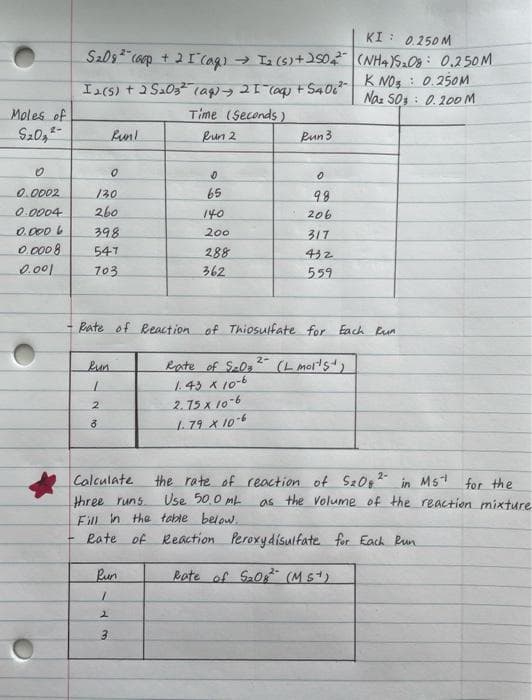
Transcribed Image Text:KI: 0.250M
3
(NH4)₂08 0.250M
KNO: 0.250M
Na: 503 0.200 M
S₂09² (oop +2 I (aq) → I₂ (s) +250₂²
Ia(s) + 2S₂03² (aq) → 2 1 (aq) + S406²
Moles of
Time (Seconds)
2-
$₂0,²-
Run!
Run 2
Run 3
0
0
0
0.0002
130
65
9.8
0.0004 260
140
206
0.0006
398
200
317
0.0008
547
288
432
0.001
703
362
559
Rate of Reaction of Thiosulfate for Each Run
Run
Rate of S₂0₂ 2-
(L mol¹5)
1.43 x 10-6
2
2.75 x 10-6
1.79 x 10-6
the rate of reaction of S₂08 2- in Mst for the
as the volume of the reaction mixture
3
Calculate
three runs Use 50,0 ML
Fill in the table below.
+
Rate of Reaction Peroxydisulfate for Each Run
Run
Rate of 5₂08² (Mst)
1
2
3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning