In iodometric titrations thiosulfate is used as titrant and this method is used to determine the organic/inorganic reductants. How starch work as an indicator in this type of titrations? Select one: a. Starch forms a complex with thiosulfate and changes color when thiosulfate (titrant) is excess after the endpoint b. Starch is not an indicator and cannot be used in redox titrations to determine the endpoint c. The oxidized form of starch is different than its reduced form so it shows different color at the endpoint d. Starch forms a complex with lodine so when there is no lodine left, color changes
In iodometric titrations thiosulfate is used as titrant and this method is used to determine the organic/inorganic reductants. How starch work as an indicator in this type of titrations? Select one: a. Starch forms a complex with thiosulfate and changes color when thiosulfate (titrant) is excess after the endpoint b. Starch is not an indicator and cannot be used in redox titrations to determine the endpoint c. The oxidized form of starch is different than its reduced form so it shows different color at the endpoint d. Starch forms a complex with lodine so when there is no lodine left, color changes
Chapter17: Complexation And Precipitation Reactions And Titrations
Section: Chapter Questions
Problem 17.42QAP
Related questions
Question
A4.
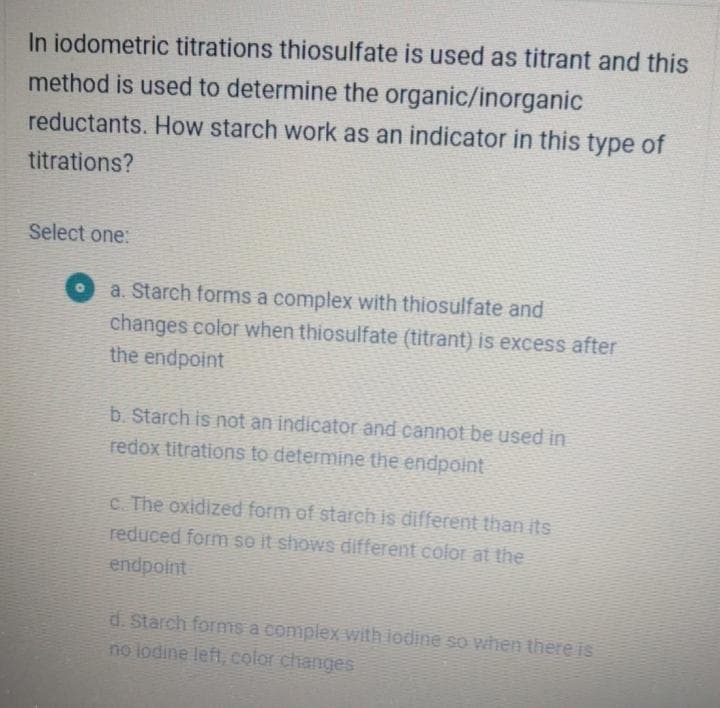
Transcribed Image Text:In iodometric titrations thiosulfate is used as titrant and this
method is used to determine the organic/inorganic
reductants. How starch work as an indicator in this type of
titrations?
Select one:
a. Starch forms a complex with thiosulfate and
changes color when thiosulfate (titrant) is excess after
the endpoint
b. Starch is not an indicator and cannot be used in
redox titrations to determine the endpoint
c. The oxidized form of starch is different than its
reduced form so it shows different color at the
endpoint
d. Starch forms a complex with iodine so when there is
no lodine left, color changes
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

