Lab Data Standardized NaOH (M) 0.4102 Initial volume of buret (mL) 0.90 Volume of vinegar (mL) 10.00 Observations The titration reaches completion at 18.3 mL. The solution remair pink for longer than 30 seconds Final volume of buret (mL) 18.30 Volume of NaOH (mL) 17.40 Molarity of acetic acid (M)
Lab Data Standardized NaOH (M) 0.4102 Initial volume of buret (mL) 0.90 Volume of vinegar (mL) 10.00 Observations The titration reaches completion at 18.3 mL. The solution remair pink for longer than 30 seconds Final volume of buret (mL) 18.30 Volume of NaOH (mL) 17.40 Molarity of acetic acid (M)
Chapter16: Applications Of Neutralization Titrations
Section: Chapter Questions
Problem 16.20QAP
Related questions
Question
Calculate the Molarity
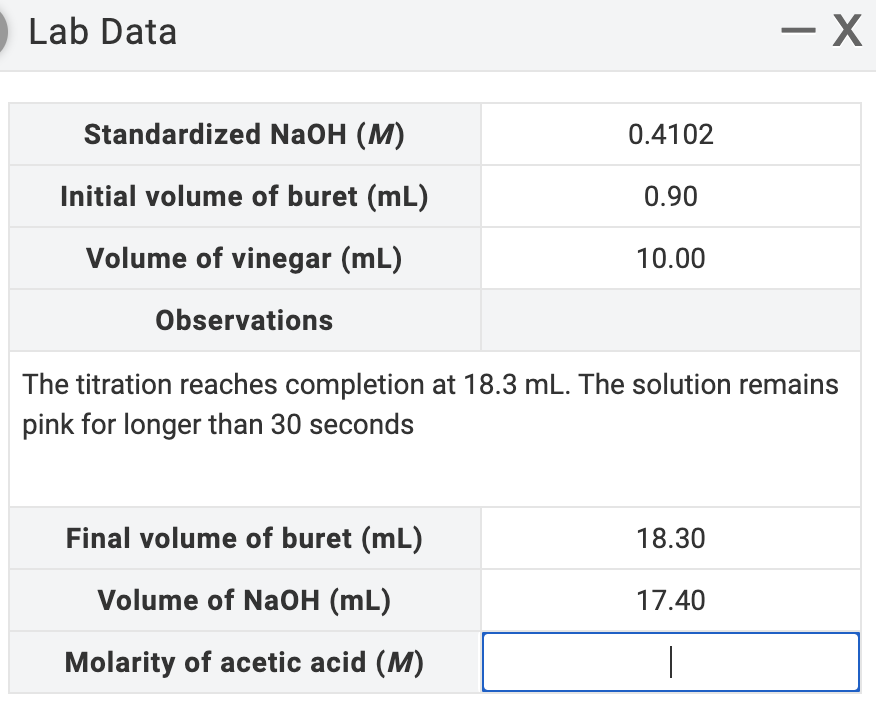
Transcribed Image Text:Lab Data
- X
Standardized NaOH (M)
0.4102
Initial volume of buret (mL)
0.90
Volume of vinegar (mL)
10.00
Observations
The titration reaches completion at 18.3 mL. The solution remains
pink for longer than 30 seconds
Final volume of buret (mL)
18.30
Volume of NaOH (mL)
17.40
Molarity of acetic acid (M)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

