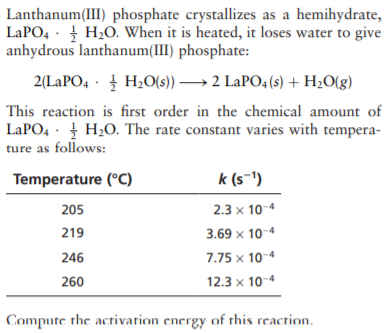
Lanthanum(III) phosphate crystallizes as a hemihydrate, LAPO4 · H20. When it is heated, it loses water to give anhydrous lanthanum(III) phosphate: 2(LAPO4 · H2O(s)) → 2 LaPO4 (s) + H20(g) This reaction is first order in the chemical amount of LAPO, · H2O. The rate constant varies with tempera- ture as follows: Temperature (°C) k (s-1) 205 2.3 x 10-4 219 3.69 x 10-4 246 7.75 x 10-4 260 12.3 x 10-4 Compute the activation energy of this reaction.
Lanthanum(III) phosphate crystallizes as a hemihydrate, LAPO4 · H20. When it is heated, it loses water to give anhydrous lanthanum(III) phosphate: 2(LAPO4 · H2O(s)) → 2 LaPO4 (s) + H20(g) This reaction is first order in the chemical amount of LAPO, · H2O. The rate constant varies with tempera- ture as follows: Temperature (°C) k (s-1) 205 2.3 x 10-4 219 3.69 x 10-4 246 7.75 x 10-4 260 12.3 x 10-4 Compute the activation energy of this reaction.
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter14: Chemical Kinetics: The Rates Of Chemical Reactions
Section: Chapter Questions
Problem 19PS: Hydrogen peroxide, H2O2(aq), decomposes to H2O() and O2(g) in a reaction that is first-order in H2O2...
Related questions
Question
Transcribed Image Text:Lanthanum(III) phosphate crystallizes as a hemihydrate,
LAPO4 · H20. When it is heated, it loses water to give
anhydrous lanthanum(III) phosphate:
2(LAPO4 · H2O(s)) → 2 LaPO4 (s) + H20(g)
This reaction is first order in the chemical amount of
LAPO, · H2O. The rate constant varies with tempera-
ture as follows:
Temperature (°C)
k (s-1)
205
2.3 x 10-4
219
3.69 x 10-4
246
7.75 x 10-4
260
12.3 x 10-4
Compute the activation energy of this reaction.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning