Le Chatelier's Principle in Iron Thiocyanate Equilibrium QUESTIONS 1. Write the equilibrium constant expression for the reaction between Fe" and SCN". 2. Based on your observation in test tube 10, is the reaction between Fe" and SCN exothermic or endothermic? 3. Write the net ionic equations of any reactions that occur in test tubes 4 to 9. (Use the table given.) 4. When equilibrium is once more established in test tube 8, how do the concentrations of Fe", SCN", and FESCN* with their concentrations before adding the Na;C,O,? compare
Le Chatelier's Principle in Iron Thiocyanate Equilibrium QUESTIONS 1. Write the equilibrium constant expression for the reaction between Fe" and SCN". 2. Based on your observation in test tube 10, is the reaction between Fe" and SCN exothermic or endothermic? 3. Write the net ionic equations of any reactions that occur in test tubes 4 to 9. (Use the table given.) 4. When equilibrium is once more established in test tube 8, how do the concentrations of Fe", SCN", and FESCN* with their concentrations before adding the Na;C,O,? compare
Chapter17: Spontaneity, Entropy, And Free Energy
Section: Chapter Questions
Problem 90AE: In the text, the equation G=G+RTIn(Q) was derived for gaseous reactions where the quantities in Q...
Related questions
Question
100%
Please answer questions 1 through 4 based on the table I filled in and provide explanations/detail when necessary! Thank you!
Transcribed Image Text:Cluudress/ Aq rons renmove thros g Shifpt H
Crar Shifts Iowareds reac tars lle
10n5, forms precip: iceauil. reactonts
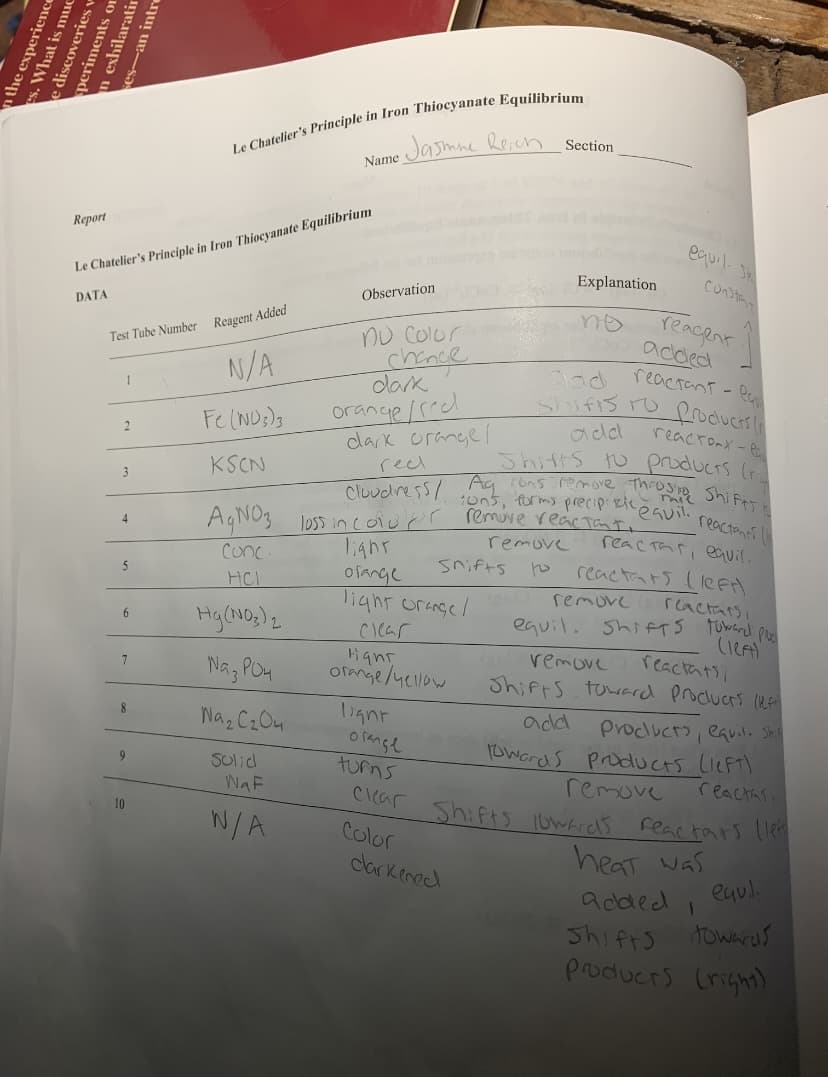
Jasmne Reich
Section
Name
Report
equil. S
Le Chatelier's Principle in Iron Thiocyanate Equilibrium
Explanation
CoNta
Observation
reacent
added
reactant - B
DATA
nu Colur
chance
dak
Test Tube Number Reagent Added
N/A
orange/reed
dark orange
Shufis ro
add
Fe (ND;)g
reacronx - P
Shifts to products (ri
KSCN
recl
3
remuve reacTanto
AGNO3 loss incoiupr
4.
reac Thri eauil.
reactars (lee)
reactars
equil. Shi FTS TUwared por
remove
Conc
Snifts
ofange
lighr ornge/
5
HCI
remuve
6.
CIcar
Hant
ofmge/yellow
reactats,
Shifts toward proclucrs (uA
remove
Nag PO4
7
Naz Cz Ou
add
Proclucro, eaいい. Sh
owords products LieFt)
reacthsi
8.
o range
turns
SUlid
WaF
remove
10
W/A
Color
heat was
eul.
darkened
added I
Shifrs
towards
producrs (right)
What is
iscoveries

Transcribed Image Text:Todine Clock
Le Chatelier's Principle in Iron Thiocyanate Equilibrium
QUESTIONS
1. Write the equilibrium constant expression for the reaction between Fe" and SCN.
2. Based on your observation in test tube 10, is the reaction between Fe" and SCN exothermic or
endothermic?
3. Write the net ionic equations of any reactions that occur in test tubes 4 to 9. (Use the table given.)
4. When equilibrium is once more established in test tube 8, how do the concentrations of Fe", SCN", and
FESCN* compare with their concentrations before adding the Na;C;O,?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning