Learning Calculate the number of pounds of CO, released into the atmosphere when a 13.0 gallon tank of gasoline is burned in an automobile engine. Assume that gasoline is primarily octane, C, H8, and that the density of gasoline is 0.692 g mL-1. This assumption ignores additives. Also, assume complete combustion.
Learning Calculate the number of pounds of CO, released into the atmosphere when a 13.0 gallon tank of gasoline is burned in an automobile engine. Assume that gasoline is primarily octane, C, H8, and that the density of gasoline is 0.692 g mL-1. This assumption ignores additives. Also, assume complete combustion.
Chapter26: Biodiesel
Section: Chapter Questions
Problem 3Q
Related questions
Question
Transcribed Image Text:Ise SEVENTH EDITION
sented by Macmillan Leaning
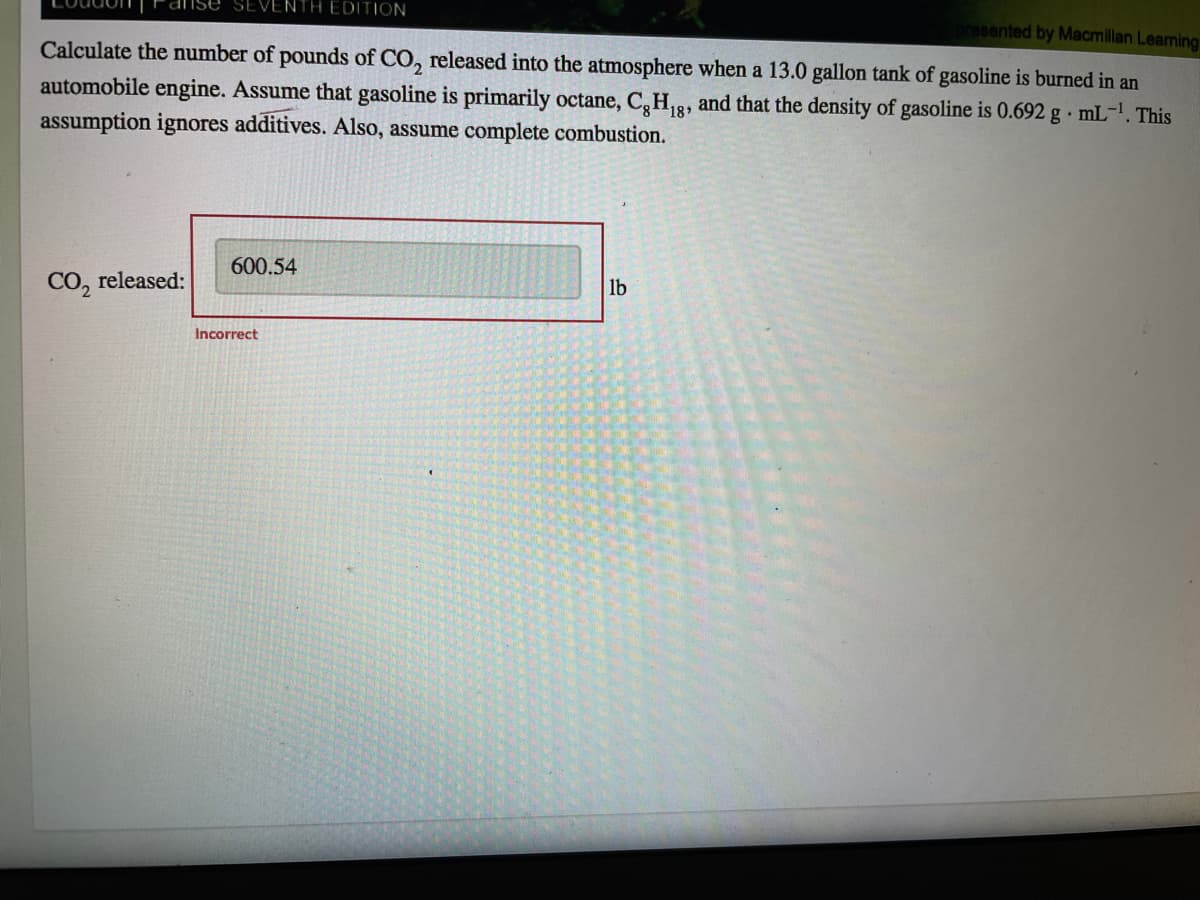
Calculate the number of pounds of CO, released into the atmosphere when a 13.0 gallon tank of gasoline is burned in an
automobile engine. Assume that gasoline is primarily octane, CaH8, and that the density of gasoline is 0.692 g · mL1. This
assumption ignores additives. Also, assume complete combustion.
600.54
CO, released:
lb
Incorrect
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning



Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning