
Methane, CH4, can be converted to methanol, which, like ethanol, can be used as a fuel. The energy level diagram shown here presents relationships between energies of the fuels and their oxidation products. Use the information in the diagram to answer the following questions. (The energy terms are per mol-rxn.)
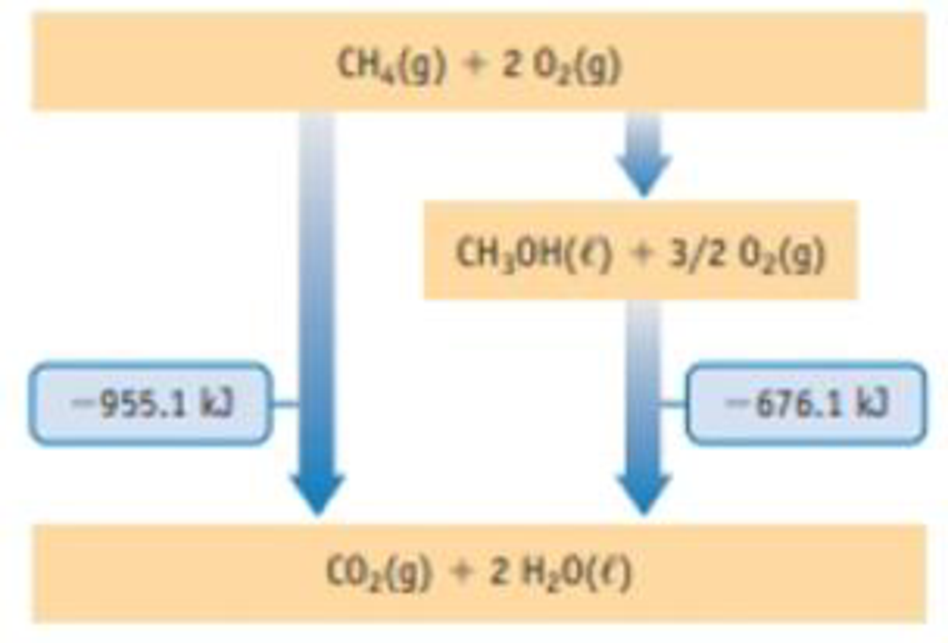
- (a) Which fuel, methanol or methane, yields the most energy per mole when burned?
- (b) Which fuel yields the most energy per gram when burned?
- (c) What is the enthalpy change for the conversion of methane to methanol by reaction with O2(g)?
- (d) Each arrow on the diagram represents a
chemical reaction . Write the equation for the reaction that converts methane to methanol.
(a)
Interpretation:
The fuel that yields the most energy per mole when burned has to be determined
Concept Introduction:
Heat energy required to raise the temperature of 1g of substance by 1K.Energy gained or lost can be calculated using the below equation.
Where, q= energy gained or lost for a given mass of substance (m), C =specific heat capacity,
The standard molar enthalpy of formation is the enthalpy change
Explanation of Solution
From the energy level diagram,
Methane liberates at
Methanol liberates at
So, methane yields most energy per mole when it’s burned.
(b)
Interpretation:
The fuel yields the most energy per gram when burned has to be calculated.
Concept Introduction:
Heat energy required to raise the temperature of 1g of substance by 1K.Energy gained or lost can be calculated using the below equation.
Where, q= energy gained or lost for a given mass of substance (m), C =specific heat capacity,
The standard molar enthalpy of formation is the enthalpy change
Explanation of Solution
The fuel yields the most energy per gram when burned
Molar mass of methane is
For methane=
Molar mass of methanol is
For methanol=
So, the fuel that yields the most energy per gram when burned is methane
(c)
Interpretation:
The enthalpy change for the conversion of methane by reaction with
Concept Introduction:
Heat energy required to raise the temperature of 1g of substance by 1K. Energy gained or lost can be calculated using the below equation.
Where, q= energy gained or lost for a given mass of substance (m), C =specific heat capacity,
The standard enthalpy change of combustion of a compound is the enthalpy change which occurs when one gram of the compound is burned completely in oxygen under standard conditions, and with everything in its standard state.
Explanation of Solution
From the energy level diagram the values obtained are
Methane liberates at
Methanol liberates at
(d)
Interpretation:
The equation for the reaction that converts methane to methanol has to be identified.
Concept Introduction:
Heat energy required to raise the temperature of 1g of substance by 1K. Energy gained or lost can be calculated using the below equation.
Where, q= energy gained or lost for a given mass of substance (m), C =specific heat capacity,
The standard enthalpy change of combustion of a compound is the enthalpy change which occurs when one gram of the compound is burned completely in oxygen under standard conditions, and with everything in its standard state.
Explanation of Solution
From the energy level diagram the obtained equation for the conversion of methane to methanol is
Want to see more full solutions like this?
Chapter 5 Solutions
Chemistry & Chemical Reactivity
- A rebreathing gas mask contains potassium superoxide, KO2, which reacts with moisture in the breath to give oxygen. 4KO2(s)+2H2O(l)4KOH(s)+3O2(g) Estimate the grams of potassium superoxide required to supply a persons oxygen needs for one hour. Assume a person requires 1.00 102 kcal of energy for this time period. Further assume that this energy can be equated to the heat of combustion of a quantity of glucose, C6H12O6, to CO2(g) and H2O(l). From the amount of glucose required to give 1.00 102 kcal of heat, calculate the amount of oxygen consumed and hence the amount of KO2 required. The ff0 for glucose(s) is 1273 kJ/mol.arrow_forwardThe equation for the fermentation of glucose to alcohol and carbon dioxide is: C6H12O6(aq) 2C2H5OH(aq) + 2CO2(g) The enthalpy change for the reaction is 67 kJ. Is this reaction exothermic or endothermic? Is energy, in the form of heat, absorbed or evolved as the reaction occurs?arrow_forwardWhen one mole of ethylene gas, C2H4, reacts with fluorine gas, hydrogen fluoride and carbon tetrafluoride gases are formed and 2496.7 kJ of heat are given off. What is Hf for CF4(g)?arrow_forward
- What mass of acetylene, C2H2(g), must be burned to produce 3420 kJ of heat, given that its enthalpy of combustion is 1301 kJ/mol? Compare this with the answer to Exercise 5.91 and determine which substance produces more heat per gram.arrow_forwardGraphite is burned in oxygen to give carbon monoxide and carbon dioxide. If the product mixture is 33% CO and 67% CO2 by mass, what is the heat from the combustion of 1.00 g of graphite?arrow_forwardBicycling Describe the energy conversions that occur when a bicyclist coasts down a long grade, then struggles to ascend a steep grade.arrow_forward
- A _________ is a device used to determine the heat associated with a chemical reaction.arrow_forwardWater gas, a mixture of carbon monoxide and hydrogen, is produced by treating carbon (in the form of coke or coal) with steam at high temperatures. (See Study Question 83.) C(s) + H2O(g) CO(g) + H2(g) Not all of the carbon available is converted to water gas since some is burned to provide the heat for the endothermic reaction of carbon and water. What mass of carbon must be burned (to CO2 gas) to provide the energy to convert 1.00 kg of carbon to water gas?arrow_forwardChlorine dioxide, ClO2, is a reddish yellow gas used in bleaching paper pulp. The average speed of a ClO2 molecule at 25C is 306 m/s. What is the kinetic energy (in joules) of a ClO2 molecule moving at this speed?arrow_forward
- Enthalpy a A 100.-g sample of water is placed in an insulated container and allowed to come to room temperature at 21C. To heat the water sample to 41C, how much heat must you add to it? b Consider the hypothetical reaction,2X(aq)+Y(l)X2Y(aq)being run in an insulated container that contains 100. g of solution. If the temperature of the solution changes from 21C to 31C, how much heat does the chemical reaction produce? How does this answer compare with that in part a? (You can assume that this solution is so dilute that it has the same heat capacity as pure water.) c If you wanted the temperature of 100. g of this solution to increase from 21C to 51C, how much heat would you have to add to it? (Try to answer this question without using a formula.) d If you had added 0.02 mol of X and 0.01 mol of Y to form the solution in part b, how many moles of X and Y would you need to bring about the temperature change described in part c. e Judging on the basis of your answers so far, what is the enthalpy of the reaction 2X(aq) + Y(l) X2Y(aq)?arrow_forwardConsider the following reaction in the vessel described in Question 57. A(g)+B(g)C(s)For this reaction, E=286 J, the piston moves up and the system absorbs 388 J of heat from its surroundings. (a) Is work done by the system? (b) How much work?arrow_forwardNatural gas companies in the United States use the therm as a unit of energy. One therm is 1105 BTU. (a) How many joules are in one therm? (1J=9.48104BTU) (b) When propane gas, C3H8, is burned in oxygen, CO2 and steam are produced. How many therms of energy are given off by 1.00 mol of propane gas?arrow_forward
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





