Learning Task 3 Determine if each of the following redox reactions is spontaneous or not. 1. Cu + 2Ag+→ Cu²+ + 2Ag 2. Al³+ + Fe → Al + Fe²+ 3. Zn+ Ni2+ Zn2+ + Ni 4. 3Pb + 2AU3+3Pb2+ + 2AU 5. Hg + Pb2+ Hg2+ + Pb Solve the following problems. 1. A voltaic cell is based on a a Co2+ /Co half-cell and an AgCI/Ag half-cell. a. What reaction occurs at the anode? b. What is the standard cell potential? 2. Using the standard reduction potentials, calculate the standard emf for each of the following reactions: a. 2Al3+ + 3Cd 2A1+3Cd²+ b. Cr+ 2Cr³+→3Cr2+ C. H₂ + 12 → 2H+ + 21- Learning Task 4
Learning Task 3 Determine if each of the following redox reactions is spontaneous or not. 1. Cu + 2Ag+→ Cu²+ + 2Ag 2. Al³+ + Fe → Al + Fe²+ 3. Zn+ Ni2+ Zn2+ + Ni 4. 3Pb + 2AU3+3Pb2+ + 2AU 5. Hg + Pb2+ Hg2+ + Pb Solve the following problems. 1. A voltaic cell is based on a a Co2+ /Co half-cell and an AgCI/Ag half-cell. a. What reaction occurs at the anode? b. What is the standard cell potential? 2. Using the standard reduction potentials, calculate the standard emf for each of the following reactions: a. 2Al3+ + 3Cd 2A1+3Cd²+ b. Cr+ 2Cr³+→3Cr2+ C. H₂ + 12 → 2H+ + 21- Learning Task 4
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter16: Thermodynamics
Section: Chapter Questions
Problem 1E: What is a spontaneous reaction?
Related questions
Question
(CQ6)
Hello! Please help me answer this one. Please refer to the given picture/s below for the questions. Please read the instructions and directions very carefully. Double and triple check your answers, previous tutors got it wrong.
NOTE: Type only your answers. Please do not handwritten your answers. Make sure your formulas, solutions and answers' format are all correct.
ANSWER LEARNING TASK 3 & 4 ONLY!
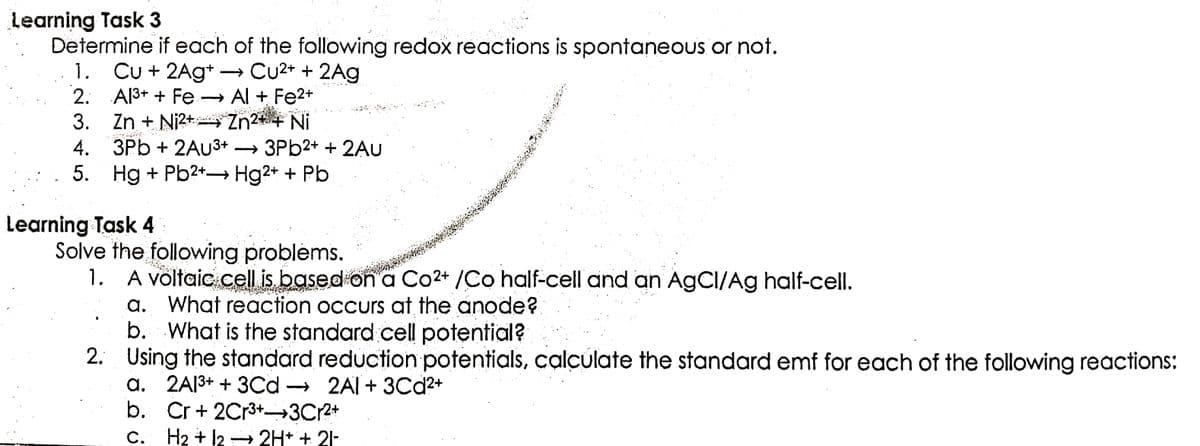
Transcribed Image Text:Learning Task 3
Determine if each of the following redox reactions is spontaneous or not.
1. Cu + 2Ag+. → Cu²+ + 2Ag
2.
Al³+ + Fe → Al + Fe²+
Zn²+ + Ni
KATA
3. Zn + Ni²+
4. 3Pb + 2AU³+ → 3Pb2+ + 2AU
5. Hg + Pb²+→ Hg2+ + Pb
Solve the following problems.
1. A voltaic cell is based on a Co2+ /Co half-cell and an AgCI/Ag half-cell.
a. What reaction occurs at the anode?
b. What is the standard cell potential?
2. Using the standard reduction potentials, calculate the standard emf for each of the following reactions:
a. 2A1³+ + 3Cd
2A1+ 3Cd²+
Cr + 2Cr³+ 3Cr²+
b.
C. H₂ + 12 → 2H+ + 21-
Learning Task 4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
FOLLOW UP QUESTION:
Hello! Thank you for your urgent answer.
Please answer the LEARNING TASK 4!
Please review and verify also your previous answers if either it's right or wrong.
Please take note to not handwritten your answer, pure typewritten only. Thank you!
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax