li 5. Percent yield calculation: a. Calculate theoretical yield: Mass unknown 1 Mol carbonate grams NaCl (molar mass) grams identity X mol NaCl grams NaCl 2.016g X Grams carbonate X mol carbonate 1 mol NaCl b. Percent yield: (X grams NaCl from experiment) * 100 = X % (X grams theoretical NaCl)
li 5. Percent yield calculation: a. Calculate theoretical yield: Mass unknown 1 Mol carbonate grams NaCl (molar mass) grams identity X mol NaCl grams NaCl 2.016g X Grams carbonate X mol carbonate 1 mol NaCl b. Percent yield: (X grams NaCl from experiment) * 100 = X % (X grams theoretical NaCl)
Chapter16: Data Processing With Excel
Section: Chapter Questions
Problem 4P
Related questions
Question
First picture is data if needed if you do more than one problem please label

Transcribed Image Text:Data: (List Data; use table if needed.)
Name
Data
Unknown number
304
Mass of evaporating dish
35.452
Mass of dish + unknown sample
37.468
Volume of HCl used
14.0
Mass NaCl + dish after first drying
37.412
Mass NaCl + dish after second drying
37.323
oratin
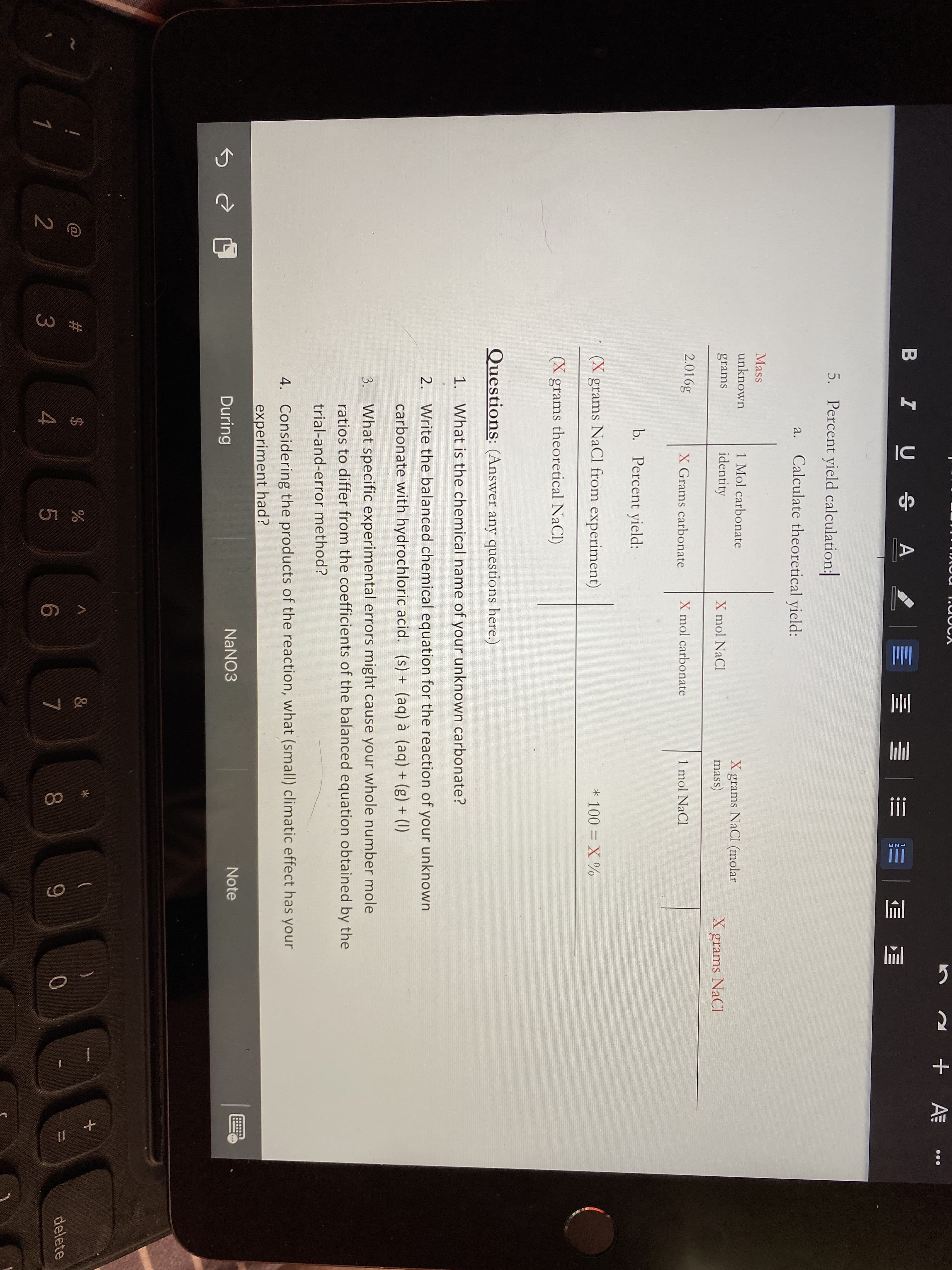
Transcribed Image Text:li
5. Percent yield calculation:
a. Calculate theoretical yield:
Mass
unknown
1 Mol carbonate
grams NaCl (molar
mass)
grams
identity
X mol NaCl
grams NaCl
2.016g
X Grams carbonate
X mol carbonate
1 mol NaCl
b. Percent yield:
(X grams NaCl from experiment)
* 100 = X %
(X grams theoretical NaCl)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

