Light of wavelengths shorter than 275 nm can be used to photodissociate the hydrogen molecule into hydrogen atoms in the gas phase. A 70.0 mL glass cylinder contains H, (g) at 65.0 mtorr and 25 °C. What minimum amount of light energy must be absorbed by the hydrogen in the tube to dissociate 26.0% of the molecules? 0.277 energy absorbed: Incorrect
Light of wavelengths shorter than 275 nm can be used to photodissociate the hydrogen molecule into hydrogen atoms in the gas phase. A 70.0 mL glass cylinder contains H, (g) at 65.0 mtorr and 25 °C. What minimum amount of light energy must be absorbed by the hydrogen in the tube to dissociate 26.0% of the molecules? 0.277 energy absorbed: Incorrect
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter4: Introduction To Quantum Mechanics
Section: Chapter Questions
Problem 50AP
Related questions
Question
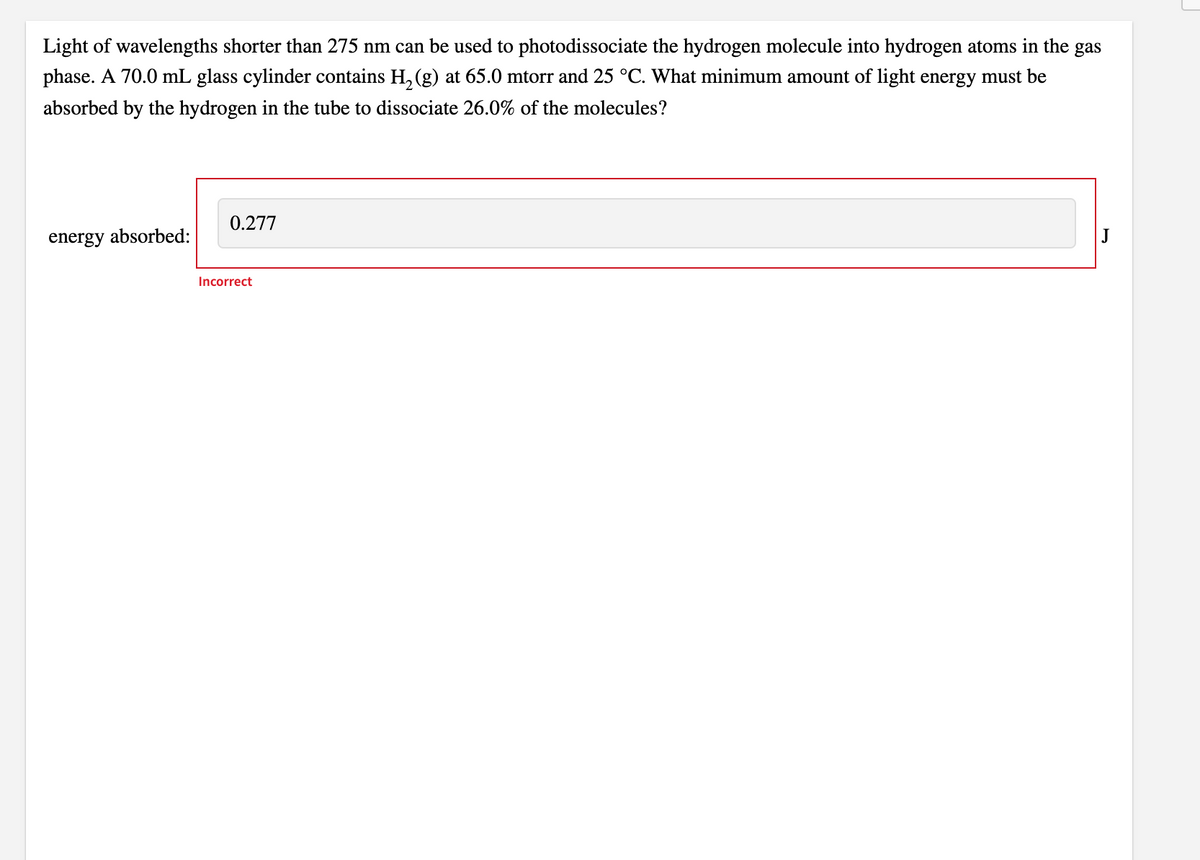
Transcribed Image Text:Light of wavelengths shorter than 275 nm can be used to photodissociate the hydrogen molecule into hydrogen atoms in the gas
phase. A 70.0 mL glass cylinder contains H, (g) at 65.0 mtorr and 25 °C. What minimum amount of light energy must be
absorbed by the hydrogen in the tube to dissociate 26.0% of the molecules?
0.277
energy absorbed:
Incorrect
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning