liquid density acetone 0.79 g em pentane 0.63 g em dimethyl sulfoxide 1.1 g-em chloroform 1.5 gem ethanolamine 1.0 gem Next, the chemist measures the volume of the unknown liquid as 0.532 L and the mass of the unknown liquid as 789. g. Calculate the density of the liquid. Be sure your answer has the correct number of significant digits. Given the data above, is it possible to identify the liquid? Oves O no acetone O pentane O dimethyl sulfoxide O chloroform ethanolamine If it is possible to identify the liquid, do so.
liquid density acetone 0.79 g em pentane 0.63 g em dimethyl sulfoxide 1.1 g-em chloroform 1.5 gem ethanolamine 1.0 gem Next, the chemist measures the volume of the unknown liquid as 0.532 L and the mass of the unknown liquid as 789. g. Calculate the density of the liquid. Be sure your answer has the correct number of significant digits. Given the data above, is it possible to identify the liquid? Oves O no acetone O pentane O dimethyl sulfoxide O chloroform ethanolamine If it is possible to identify the liquid, do so.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter1: Chemistry: An Introduction
Section: Chapter Questions
Problem 6ALQ: Scientific models do not describe reality. They are simplifications aid therefore incorrect at some...
Related questions
Question
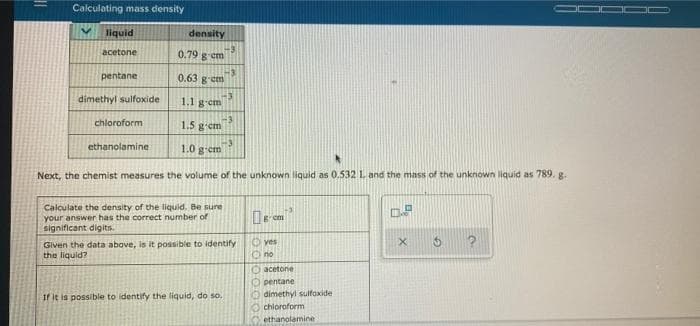
Transcribed Image Text:Calculating mass density
liquid
density
acetone
0.79 g em
pentane
0.63 gem
dimethyl sulfoxide
1.1 g-em
chloroform
1.5 g-em
ethanolamine
1.0 g-em
Next, the chemist measures the volume of the unknown liquid as 0.532 L and the mass of the unknown liquid as 789. g.
Calculate the density of the liquid. Be sure
your answer has the correct number of
significant digits.
yes
Given the data above, is it possible to identify
the liquid?
Ono
Oacetone
O pentane
O dimethyl sulfoxide
O chloroform
If it is possible to identify the liquid, do so.
ethanolamine
%3D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
