Next, the chemist measures the volume of the unknown liquid as 1584. cm and the mass of the unknown liquid as 1.48 kg. Calculate the density of the liquid. Be sure your answer has the correct number of significant digits. -- g•mL Given the data above, is it possible to identify the liquid? O yes O no O ethanolamine O carbon tetrachloride O methyl acetate O dimethyl sulfoxide O acetone If it is possible to identify the liquid, do so.
Next, the chemist measures the volume of the unknown liquid as 1584. cm and the mass of the unknown liquid as 1.48 kg. Calculate the density of the liquid. Be sure your answer has the correct number of significant digits. -- g•mL Given the data above, is it possible to identify the liquid? O yes O no O ethanolamine O carbon tetrachloride O methyl acetate O dimethyl sulfoxide O acetone If it is possible to identify the liquid, do so.
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter2: Matter And Energy
Section: Chapter Questions
Problem 7E: 7.The word pour is commonly used in reference to liquids but not to solids or gases. Can you pour a...
Related questions
Question
100%
need assistance
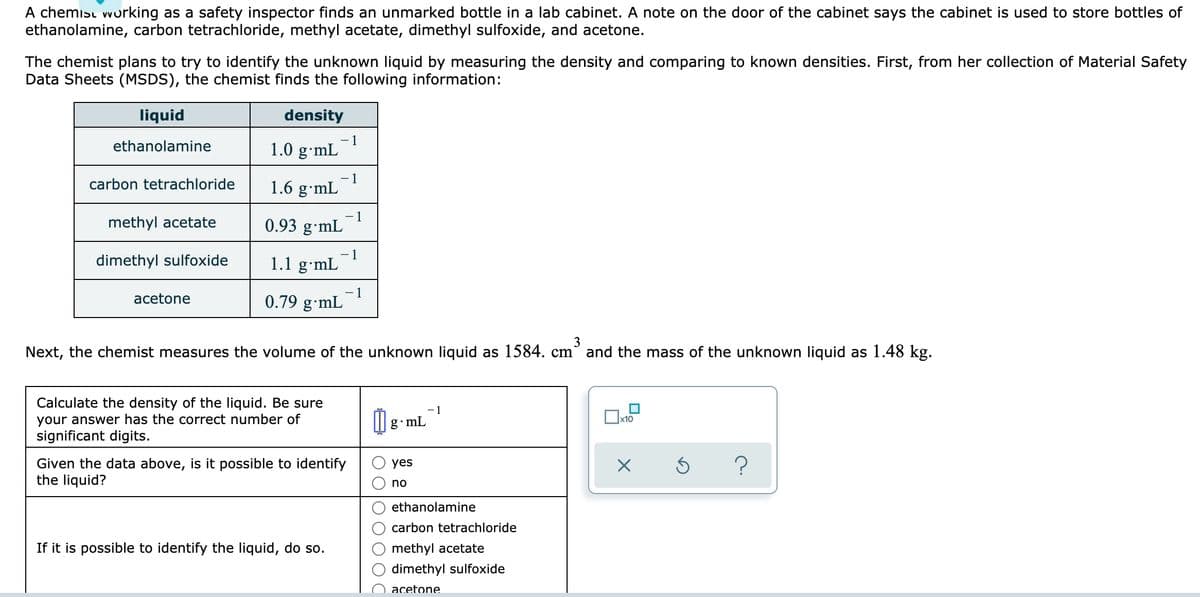
Transcribed Image Text:A chemisı vworking as a safety inspector finds an unmarked bottle in a lab cabinet. A note on the door of the cabinet says the cabinet is used to store bottles of
ethanolamine, carbon tetrachloride, methyl acetate, dimethyl sulfoxide, and acetone.
The chemist plans to try to identify the unknown liquid by measuring the density and comparing to known densities. First, from her collection of Material Safety
Data Sheets (MSDS), the chemist finds the following information:
liquid
density
1
1.0 g·mL
ethanolamine
1
1.6 g•mL
carbon tetrachloride
methyl acetate
- 1
0.93 g•mL
dimethyl sulfoxide
1
1.1 g·mL
- 1
0.79 g•mL
acetone
3
Next, the chemist measures the volume of the unknown liquid as 1584. cm° and the mass of the unknown liquid as 1.48 kg.
Calculate the density of the liquid. Be sure
your answer has the correct number of
significant digits.
-1
g mL
Given the data above, is it possible to identify
the liquid?
yes
no
ethanolamine
carbon tetrachloride
If it is possible to identify the liquid, do so.
methyl acetate
dimethyl sulfoxide
acetone
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning