List the following atoms in order of increasing size (atomic radius): Pb, Rn, Ba. A) Rn < Pb < Ba B) Rn < Ba < Pb C) Ba < Pb < Rn D) Pb < Rn < Ba
List the following atoms in order of increasing size (atomic radius): Pb, Rn, Ba. A) Rn < Pb < Ba B) Rn < Ba < Pb C) Ba < Pb < Rn D) Pb < Rn < Ba
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter5: Electron Configurations And The Periodic Table
Section: Chapter Questions
Problem 113QRT
Related questions
Question
Transcribed Image Text:(61,450 unread) - lorenalongor x
101 Chem101
Q Fichas de aprendizaje Chem 10 x +
G In a ground state atom of Kr how many electrons total will have the quantum number { = 2?
M
Apps
G
M Gmail
YouTube
Maps
а АМAZON
Translate
O Gflights
Case Status Onlin...
b Homework Help a...
C Get Homework He...
Reading List
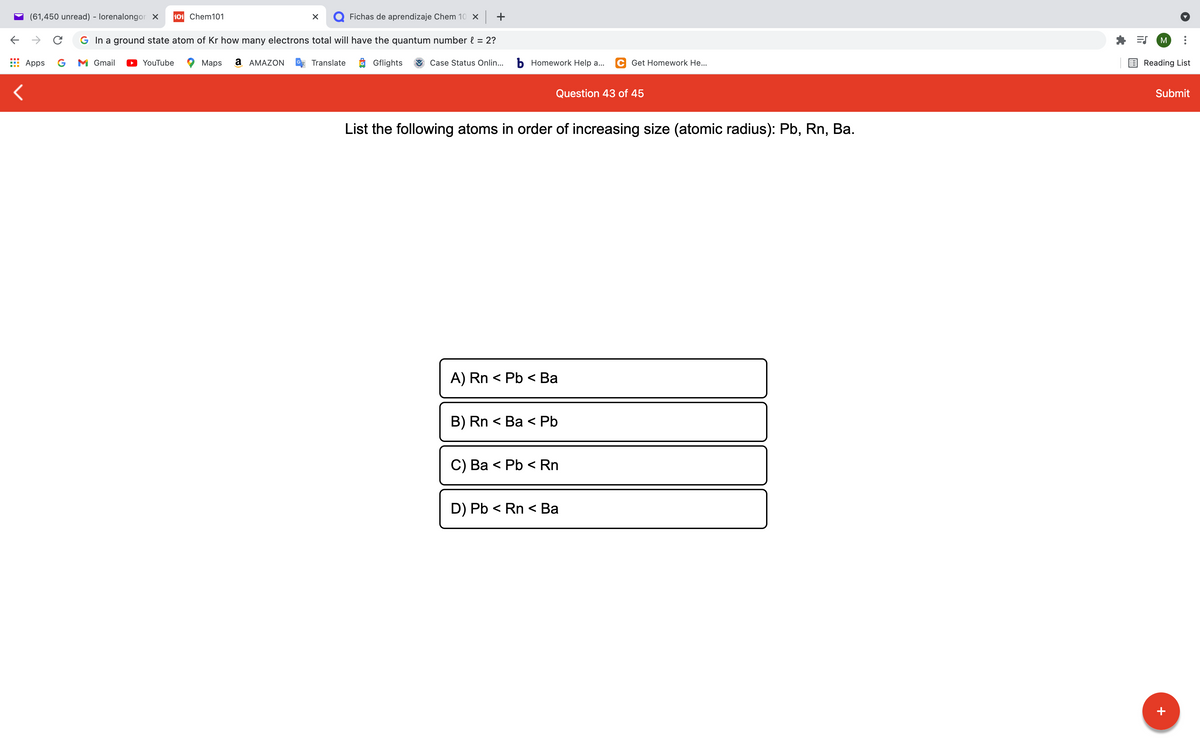
Question 43 of 45
Submit
List the following atoms in order of increasing size (atomic radius): Pb, Rn, Ba.
A) Rn < Pb < Ba
B) Rn < Ba < Pb
C) Ва < Pb < Rn
D) Pb < Rn < Ba
+
Transcribed Image Text:(61,450 unread) - lorenalongor x
101 Chem101
Q Fichas de aprendizaje Chem 10 x +
G In a ground state atom of Kr how many electrons total will have the quantum number { = 2?
M
Apps
G
M Gmail
YouTube
Maps
а АМAZON
Translate
9 Gflights
Case Status Onlin...
b Homework Help a...
C Get Homework He...
Reading List
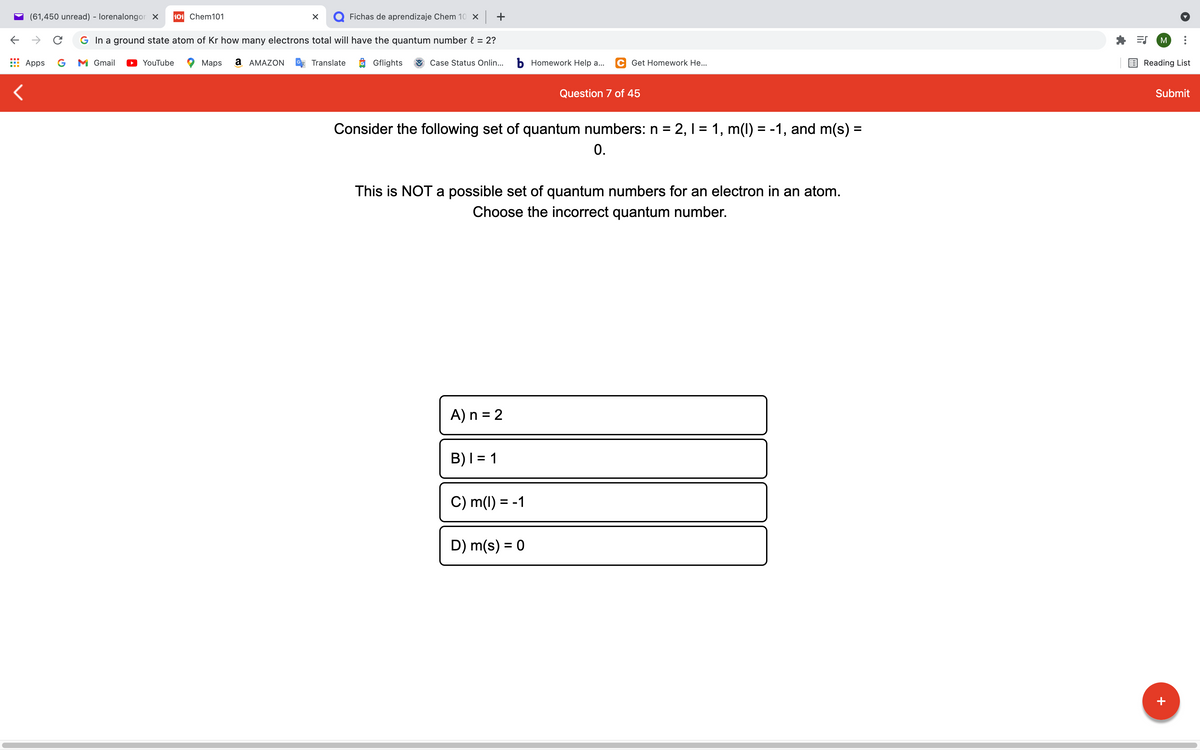
Question 7 of 45
Submit
Consider the following set of quantum numbers: n = 2, 1 = 1, m(1) = -1, and m(s)% =
0.
%3D
This is NOT a possible set of quantum numbers for an electron in an atom.
Choose the incorrect quantum number.
A) n = 2
B)I = 1
%3D
C) m(1) = -1
D) m(s) = 0
%3D
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning