Listed below are two organic acids and their pKa values. Calculate the acid dissociation constant, Ka, for each and indicate which of the two is the stronger acid. HC¬H5O2 pKa = 4.20 HCSH9O2 pka = 5.03 %3D
Listed below are two organic acids and their pKa values. Calculate the acid dissociation constant, Ka, for each and indicate which of the two is the stronger acid. HC¬H5O2 pKa = 4.20 HCSH9O2 pka = 5.03 %3D
Chapter20: Carboxylic Acids And Nitriles
Section20.4: Substituent Effects On Acidity
Problem 7P: Dicarboxylic acids have two dissociation constants, one for the initial dissociation into a...
Related questions
Question
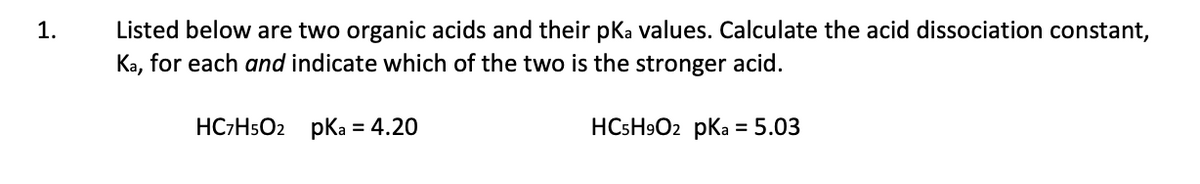
Transcribed Image Text:Listed below are two organic acids and their pKa values. Calculate the acid dissociation constant,
Ka, for each and indicate which of the two is the stronger acid.
1.
HC-HsO2 pKa = 4.20
HCSH9O2 pKa = 5.03
%3D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning
