Write the equilibrium-constant expressions and obtain numerical values for each constant in a. the basic dissociation of ethyl amine, C2H5NH2. b. the acidic dissociation of hydrogen cyanide, HCN. c. the acidic dissociation of pyridine hydrochloride, C5H5NHC1.
Write the equilibrium-constant expressions and obtain numerical values for each constant in a. the basic dissociation of ethyl amine, C2H5NH2. b. the acidic dissociation of hydrogen cyanide, HCN. c. the acidic dissociation of pyridine hydrochloride, C5H5NHC1.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 12.65PAE
Related questions
Question
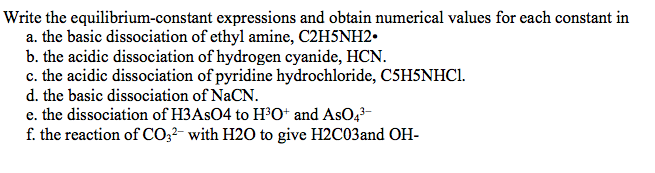
Transcribed Image Text:Write the equilibrium-constant expressions and obtain numerical values for each constant in
a. the basic dissociation of ethyl amine, C2H5NH2.
b. the acidic dissociation of hydrogen cyanide, HCN.
c. the acidic dissociation of pyridine hydrochloride, C5H5NHC1.
d. the basic dissociation of NaCN.
e. the dissociation of H3ASO4 to H³O* and AsO,-
f. the reaction of CO;2- with H2O to give H2C03and OH-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning