Lithium nitride reacts with water to produce ammonia and lithium hydroxide according to the equation Li3 N(s)3 H2 O() NH3 (g)3 LiOH(aq) Heavy water is water with the isotope deuterium in place of ordinary hydrogen, and its formula is D,O. The same reaction can be used to produce heavy ammonia, ND, (g), according to the equation Li3 N(s)+3 D20()-ND, (g)3 LiOD(aq) Calculate how many grams of heavy water are required to produce 125.0 mg ND3 (g) The mass of deuterium, D, is 2.014 g/mol g D20 mass:
Lithium nitride reacts with water to produce ammonia and lithium hydroxide according to the equation Li3 N(s)3 H2 O() NH3 (g)3 LiOH(aq) Heavy water is water with the isotope deuterium in place of ordinary hydrogen, and its formula is D,O. The same reaction can be used to produce heavy ammonia, ND, (g), according to the equation Li3 N(s)+3 D20()-ND, (g)3 LiOD(aq) Calculate how many grams of heavy water are required to produce 125.0 mg ND3 (g) The mass of deuterium, D, is 2.014 g/mol g D20 mass:
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter4: Stoichiometry
Section: Chapter Questions
Problem 4.75PAE
Related questions
Question
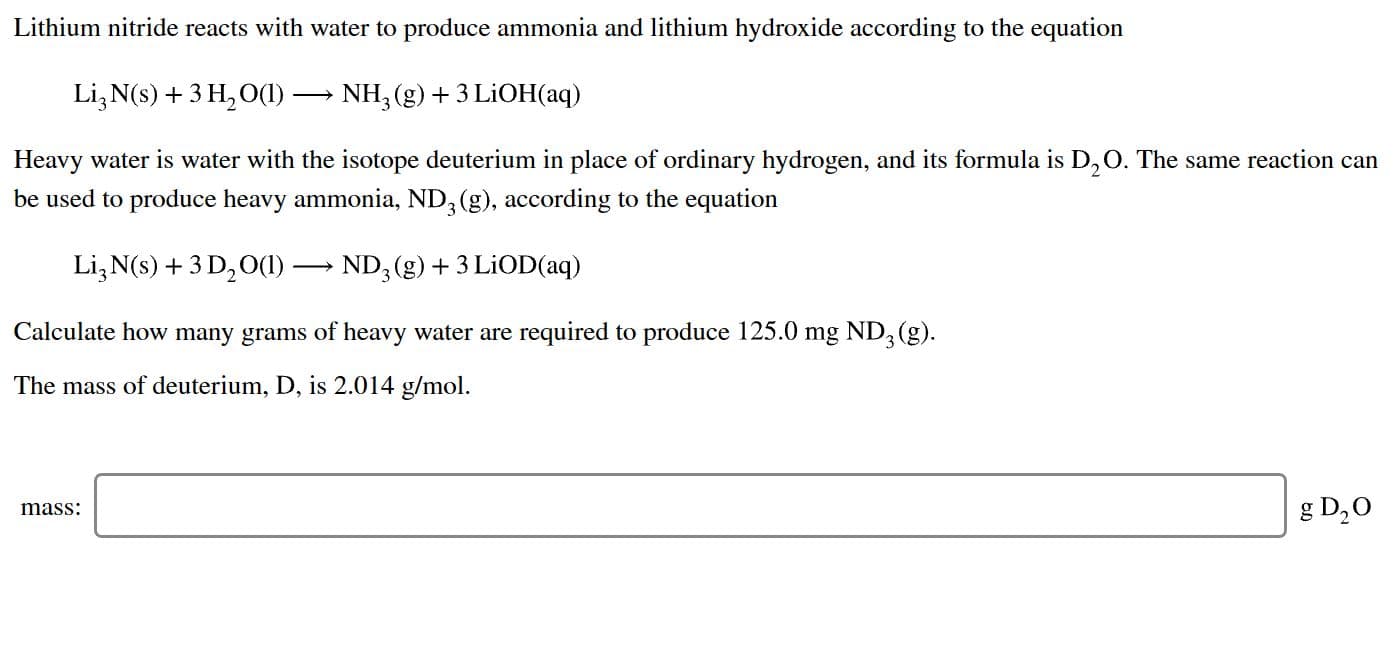
Transcribed Image Text:Lithium nitride reacts with water to produce ammonia and lithium hydroxide according to the equation
Li3 N(s)3 H2 O()
NH3 (g)3 LiOH(aq)
Heavy water is water with the isotope deuterium in place of ordinary hydrogen, and its formula is D,O. The same reaction can
be used to produce heavy ammonia, ND, (g), according to the equation
Li3 N(s)+3 D20()-ND, (g)3 LiOD(aq)
Calculate how many grams of heavy water are required to produce 125.0 mg ND3 (g)
The mass of deuterium, D, is 2.014 g/mol
g D20
mass:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 3 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning