lodine pentoxide is used as a reagent to determine the amount of carbon monoxide present in a gaseous sample. The sample is passed over heated iodine pentoxide. The products of this process are carbon dioxide and iodine. The iodine formed is extracted and added to an excess of sodium thiosulfate solution of known concentration. The remaining sodium thiosulfate is then determined by titration with a solution of iodine of known concentration. 1₂(aq) + 2S₂0₂²-(aq) → 21-(aq) + S₂O²(aq) In an analysis, a 2.00 m³ sample of gas was used and the resultant iodine extracted and added to 20 cm³ of a 0.0400 mol dm-³ solution of sodium thiosulfate, an excess. The resultant solution was then titrated against a solution of iodine of concentration 0.0100 mol dm-³. The volume of iodine solution required for complete reaction was 21.60 cm³.
lodine pentoxide is used as a reagent to determine the amount of carbon monoxide present in a gaseous sample. The sample is passed over heated iodine pentoxide. The products of this process are carbon dioxide and iodine. The iodine formed is extracted and added to an excess of sodium thiosulfate solution of known concentration. The remaining sodium thiosulfate is then determined by titration with a solution of iodine of known concentration. 1₂(aq) + 2S₂0₂²-(aq) → 21-(aq) + S₂O²(aq) In an analysis, a 2.00 m³ sample of gas was used and the resultant iodine extracted and added to 20 cm³ of a 0.0400 mol dm-³ solution of sodium thiosulfate, an excess. The resultant solution was then titrated against a solution of iodine of concentration 0.0100 mol dm-³. The volume of iodine solution required for complete reaction was 21.60 cm³.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 80AP
Related questions
Question
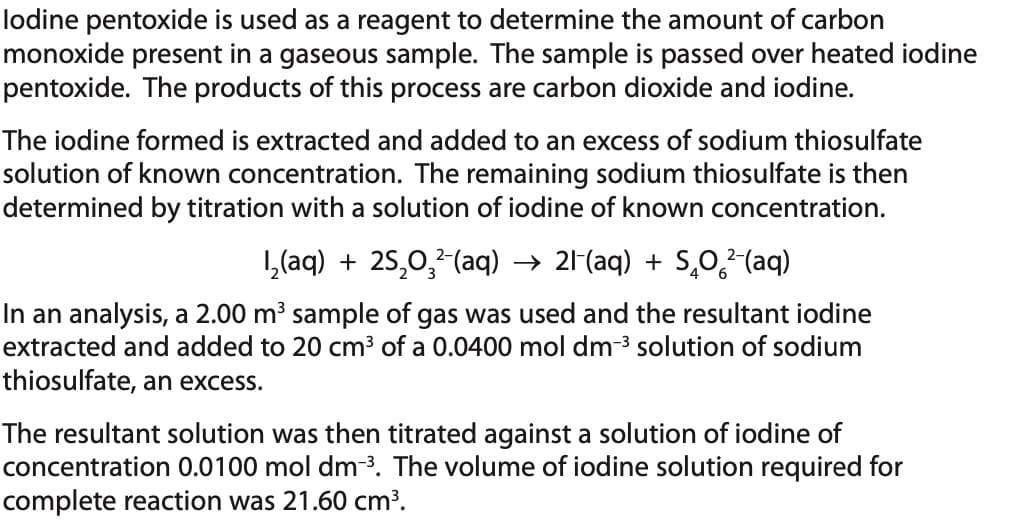
Transcribed Image Text:lodine pentoxide is used as a reagent to determine the amount of carbon
monoxide present in a gaseous sample. The sample is passed over heated iodine
pentoxide. The products of this process are carbon dioxide and iodine.
The iodine formed is extracted and added to an excess of sodium thiosulfate
solution of known concentration. The remaining sodium thiosulfate is then
determined by titration with a solution of iodine of known concentration.
1₂(aq) + 25₂0₂²-(aq) → 21-(aq) + SÃO²-(aq)
In an analysis, a 2.00 m³ sample of gas was used and the resultant iodine
extracted and added to 20 cm³ of a 0.0400 mol dm-³ solution of sodium
thiosulfate, an excess.
The resultant solution was then titrated against a solution of iodine of
concentration 0.0100 mol dm-³. The volume of iodine solution required for
complete reaction was 21.60 cm³.
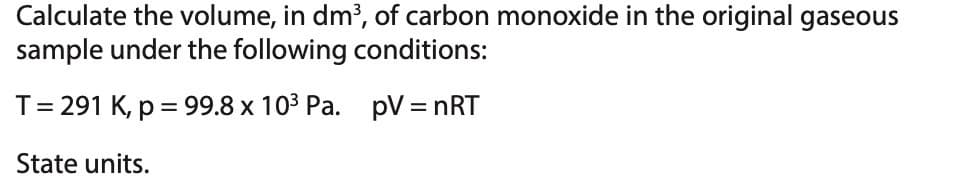
Transcribed Image Text:Calculate the volume, in dm³, of carbon monoxide in the original gaseous
sample under the following conditions:
T = 291 K, p = 99.8 x 10³ Pa. pV = nRT
State units.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning
