Lycopene (C40H56) Is an antioxidant molecule that is minimum daily intake of 6.5 mg. a. Determine the empirical formula for lycopene. (Text Text b. How many moles of lycopene are required to meet the minimum daily dose?
Lycopene (C40H56) Is an antioxidant molecule that is minimum daily intake of 6.5 mg. a. Determine the empirical formula for lycopene. (Text Text b. How many moles of lycopene are required to meet the minimum daily dose?
Essentials of Pharmacology for Health Professions
7th Edition
ISBN:9781305441620
Author:WOODROW
Publisher:WOODROW
ChapterCRE1: Comprehensive Review Exam For Part 1
Section: Chapter Questions
Problem 51CEP1
Related questions
Question
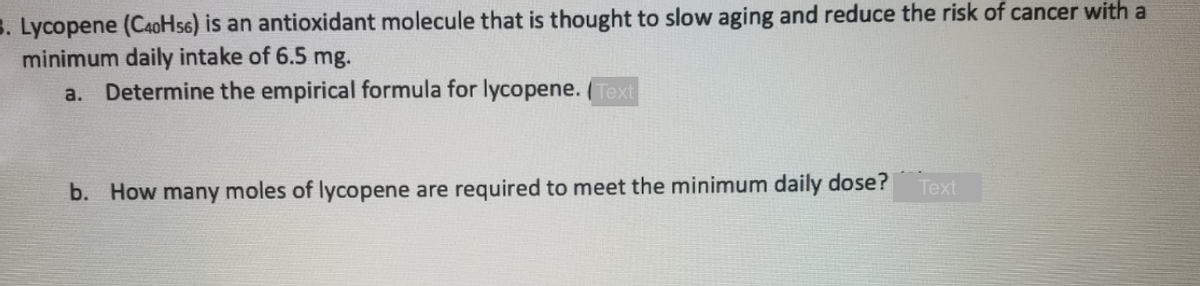
Transcribed Image Text:3. Lycopene (CaoHs6) is an antioxidant molecule that is thought to slow aging and reduce the risk of cancer with a
minimum daily intake of 6.5 mg.
Determine the empirical formula for lycopene. ( Text
a.
Тext
b. How many moles of lycopene are required to meet the minimum daily dose?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Recommended textbooks for you

Essentials of Pharmacology for Health Professions
Nursing
ISBN:
9781305441620
Author:
WOODROW
Publisher:
Cengage


Essentials of Pharmacology for Health Professions
Nursing
ISBN:
9781305441620
Author:
WOODROW
Publisher:
Cengage
