m-Nitromethyl benzoate: Put in the molecular formula of the ions responsible for peaks at 135 and 150. Use the following order: C, H, O, N and include a '+' at the end. A formatting example for mass of 44 would be C2H40+ 100- 80- 60- 40 20- 75 100 125 150 175 - 135 150
m-Nitromethyl benzoate: Put in the molecular formula of the ions responsible for peaks at 135 and 150. Use the following order: C, H, O, N and include a '+' at the end. A formatting example for mass of 44 would be C2H40+ 100- 80- 60- 40 20- 75 100 125 150 175 - 135 150
Chapter12: Structure Determination: Mass Spectrometry And Infrared Spectroscopy
Section12.SE: Something Extra
Problem 18AP: In light of the nitrogen rule mentioned in Problem 12-17, what is the molecular formula of pyridine,...
Related questions
Question
7
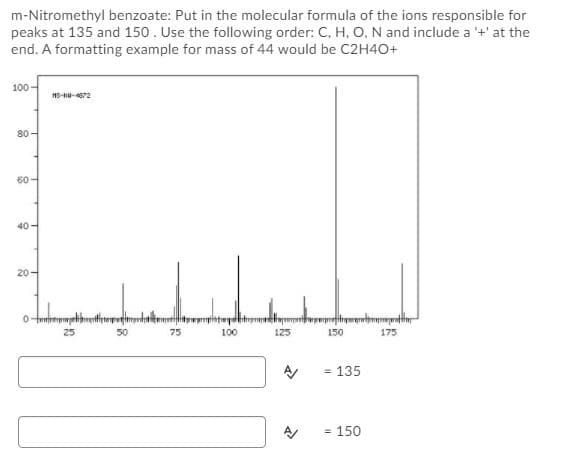
Transcribed Image Text:m-Nitromethyl benzoate: Put in the molecular formula of the ions responsible for
peaks at 135 and 150. Use the following order: C, H, O, N and include a '+' at the
end. A formatting example for mass of 44 would be C2H40+
100
80-
60-
40-
20-
25
75
100
125
150
175
= 135
= 150
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

