Make a graph a titration curve for the titration of 30.00 mL of a 0.125 M solution of acetic acid, HC2H3O2, with 0.125 M sodium hydroxide, NaOH. This does not need to be an actual graph, just a free-hand sketch. However, the sketch must include calculated values for the following points (and you must show your work). Use Appendix A.3, lonization Constants for Acids and Bases from our textbook, Interactive General Chemistry: Atoms First, for any weak acid or weak base ionization constants you need to work through this Learning Target. You can access the Appendix in the eBook on Achieve or here: 1, The initial pH of the analyte solution 2, The pH of the analyte solution after the addition of 10.00 mL of titrant 3, The pH of the analyte solution at the half-equivalence point 4,The pH of the analyte solution at the equivalence point
Make a graph a titration curve for the titration of 30.00 mL of a 0.125 M solution of acetic acid, HC2H3O2, with 0.125 M sodium hydroxide, NaOH. This does not need to be an actual graph, just a free-hand sketch. However, the sketch must include calculated values for the following points (and you must show your work). Use Appendix A.3, lonization Constants for Acids and Bases from our textbook, Interactive General Chemistry: Atoms First, for any weak acid or weak base ionization constants you need to work through this Learning Target. You can access the Appendix in the eBook on Achieve or here: 1, The initial pH of the analyte solution 2, The pH of the analyte solution after the addition of 10.00 mL of titrant 3, The pH of the analyte solution at the half-equivalence point 4,The pH of the analyte solution at the equivalence point
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter14: Equilibria In Acid-base Solutions
Section: Chapter Questions
Problem 47QAP: A 0.4000 M solution of nitric acid is used to titrate 50.00 mL of 0.237 M barium hydroxide. (Assume...
Related questions
Question
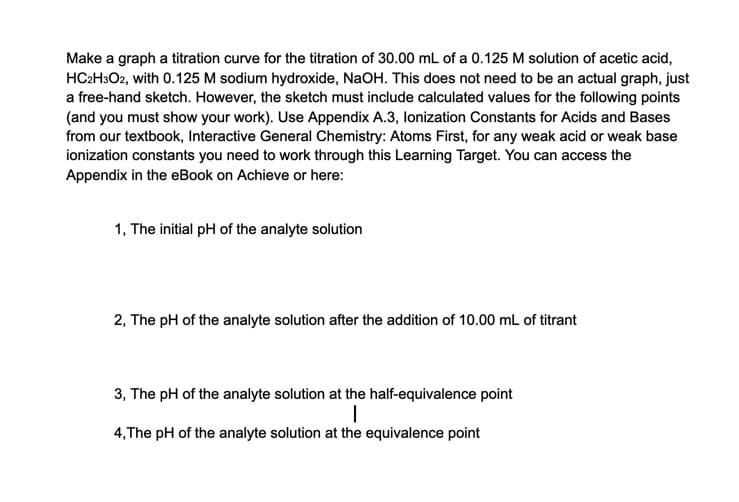
Transcribed Image Text:Make a graph a titration curve for the titration of 30.00 mL of a 0.125 M solution of acetic acid,
HC2H3O2, with 0.125 M sodium hydroxide, NaOH. This does not need to be an actual graph, just
a free-hand sketch. However, the sketch must include calculated values for the following points
(and you must show your work). Use Appendix A.3, lonization Constants for Acids and Bases
from our textbook, Interactive General Chemistry: Atoms First, for any weak acid or weak base
ionization constants you need to work through this Learning Target. You can access the
Appendix in the eBook on Achieve or here:
1, The initial pH of the analyte solution
2, The pH of the analyte solution after the addition of 10.00 mL of titrant
3, The pH of the analyte solution at the half-equivalence point
4,The pH of the analyte solution at the equivalence point
Expert Solution
Step by step
Solved in 10 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning