Many mining operations produce tailings that can be oxidized to form acids, including sulfuric acid. One chemical that reacts in this way is chalcopyrite, CuFeS2, whose net oxidation can be described by the following equation. 4 CuFeS2(s) + 17 O2(9) + 10 H20(!) → 4 Fe (OH), (s) + 4 CUSO,(aq) + 42SO4(aq) In a laboratory experiment to study weathering, a mining engineer put a 1.67 kg sample of CUFES2(s) near an empty 40 L container. Simulated weathering experiments were conducted and the runoff was collected, leading to 35.4 L of solution. A 25.00 mL aliquot of this solution was analyzed with 0.0100 M NaOH(aq). The titration required 37.3 mL of base. (a) What was the concentration (in M) of sulfuric acid? (b) Assuming the only acid in the container comes from the reacted chalcopyrite, what mass (in g) of the chalcopyrite has reacted? (c) What percentage of the chalcopyrite reacted in this experiment?
Many mining operations produce tailings that can be oxidized to form acids, including sulfuric acid. One chemical that reacts in this way is chalcopyrite, CuFeS2, whose net oxidation can be described by the following equation. 4 CuFeS2(s) + 17 O2(9) + 10 H20(!) → 4 Fe (OH), (s) + 4 CUSO,(aq) + 42SO4(aq) In a laboratory experiment to study weathering, a mining engineer put a 1.67 kg sample of CUFES2(s) near an empty 40 L container. Simulated weathering experiments were conducted and the runoff was collected, leading to 35.4 L of solution. A 25.00 mL aliquot of this solution was analyzed with 0.0100 M NaOH(aq). The titration required 37.3 mL of base. (a) What was the concentration (in M) of sulfuric acid? (b) Assuming the only acid in the container comes from the reacted chalcopyrite, what mass (in g) of the chalcopyrite has reacted? (c) What percentage of the chalcopyrite reacted in this experiment?
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter3: Chemical Reactions
Section: Chapter Questions
Problem 94QRT
Related questions
Question
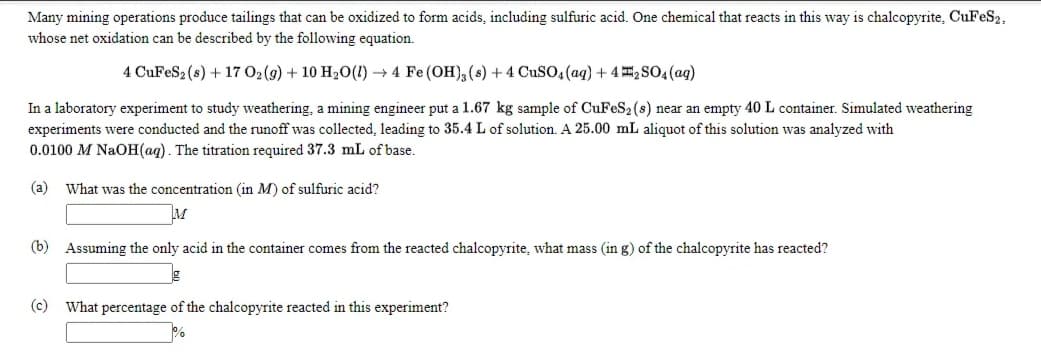
Transcribed Image Text:Many mining operations produce tailings that can be oxidized to form acids, including sulfuric acid. One chemical that reacts in this way is chalcopyrite, CuFeS2,
whose net oxidation can be described by the following equation.
4 CuFeS2 (s) + 17 02(g) + 10 H20(!) → 4 Fe (OH), (s) + 4 CuSO4 (aq) + 4I,SO4(ag)
In a laboratory experiment to study weathering, a mining engineer put a l1.67 kg sample of CuFeS2 (s) near an empty 40 L container. Simulated weathering
experiments were conducted and the runoff was collected, leading to 35.4 L of solution. A 25.00 mL aliquot of this solution was analyzed with
0.0100 M NaOH(aq). The titration required 37.3 mL of base.
(a) What was the concentration (in M) of sulfuric acid?
(b) Assuming the only acid in the container comes from the reacted chalcopyrite, what mass (in g) of the chalcopyrite has reacted?
(c) What percentage of the chalcopyrite reacted in this experiment?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning