When potassium dichromate is added to concentrated hydrochloric acid, it reacts according to the following chemical equation K,C»O¬(s) + 14 HCI(aq) → 2 K* (aq) + 2 Cr³* (aq) + 8 CI¯(aq) + 7 H20(e) + 3 Cl2(g) producing a mixed solution of chromium(III) chloride and potassium chloride and evolving gaseous chlorine. Suppose that 6.20 g of K,Cr,O, reacts with concentrated HCI, and that the final volume of the solution is 100.0 mL. Calculate the final con- centration of Cr³+(aq) and the number of moles of chlorine produced.
When potassium dichromate is added to concentrated hydrochloric acid, it reacts according to the following chemical equation K,C»O¬(s) + 14 HCI(aq) → 2 K* (aq) + 2 Cr³* (aq) + 8 CI¯(aq) + 7 H20(e) + 3 Cl2(g) producing a mixed solution of chromium(III) chloride and potassium chloride and evolving gaseous chlorine. Suppose that 6.20 g of K,Cr,O, reacts with concentrated HCI, and that the final volume of the solution is 100.0 mL. Calculate the final con- centration of Cr³+(aq) and the number of moles of chlorine produced.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter4: Chemical Reactions
Section: Chapter Questions
Problem 4.116QP: Bone was dissolved in hydrochloric acid, giving 50.0 mL of solution containing calcium chloride,...
Related questions
Question
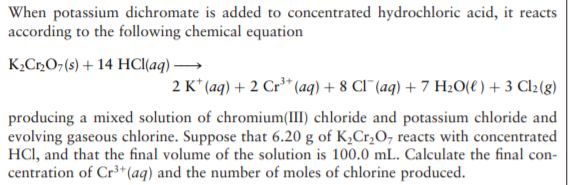
Transcribed Image Text:When potassium dichromate is added to concentrated hydrochloric acid, it reacts
according to the following chemical equation
K,C»O¬(s) + 14 HCI(aq) →
2 K* (aq) + 2 Cr³* (aq) + 8 CI¯(aq) + 7 H20(e) + 3 Cl2(g)
producing a mixed solution of chromium(III) chloride and potassium chloride and
evolving gaseous chlorine. Suppose that 6.20 g of K,Cr,O, reacts with concentrated
HCI, and that the final volume of the solution is 100.0 mL. Calculate the final con-
centration of Cr³+(aq) and the number of moles of chlorine produced.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning