Mass of iron 64.8973 g Mass of water 61.9272 g Initial temperature of iron (boiling water Tm) 98.0 °C Initial temperature of calorimeter + water, T; 22.1 °C Final temperature, T¡ 30.3 °С TEMPERATURE CHANGE AT for iron, °C -67.7 AT for water, °C 8.2 НЕАТ The specific heat of water is 4.184 J g- K-1. • The specific heat of iron is 0.450 J g-1K-!. Unrounded Rounded Fe. () -1.98 x 103 1977.4 (x -1977.10 (V 2.1 x 103 2124.7 X 2124.65 Iwater, () 47541.3005 (x -147.55 -148 10510 40
Mass of iron 64.8973 g Mass of water 61.9272 g Initial temperature of iron (boiling water Tm) 98.0 °C Initial temperature of calorimeter + water, T; 22.1 °C Final temperature, T¡ 30.3 °С TEMPERATURE CHANGE AT for iron, °C -67.7 AT for water, °C 8.2 НЕАТ The specific heat of water is 4.184 J g- K-1. • The specific heat of iron is 0.450 J g-1K-!. Unrounded Rounded Fe. () -1.98 x 103 1977.4 (x -1977.10 (V 2.1 x 103 2124.7 X 2124.65 Iwater, () 47541.3005 (x -147.55 -148 10510 40
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 30E: When 1.0 g of fructose, C6H12O6(s), a sugar commonly found in fruits, is burned in oxygen in a bomb...
Related questions
Question
help me find the heat capacity and the adjusted heat capacity with the unrounded and rounded values for both
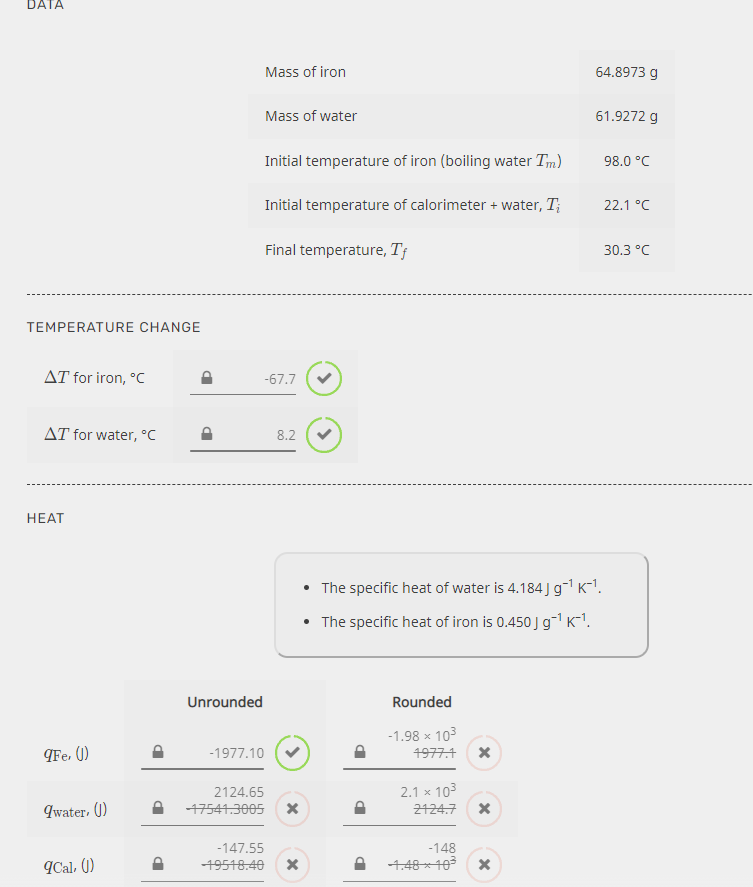
Transcribed Image Text:DATA
Mass of iron
64.8973 g
Mass of water
61.9272 g
Initial temperature of iron (boiling water Tm)
98.0 °C
Initial temperature of calorimeter + water, T;
22.1 °C
Final temperature, T¡
30.3 °C
TEMPERATURE CHANGE
AT for iron, °C
-67.7
AT for water, °c
8.2
НЕАТ
The specific heat of water is 4.184 J g-1 K-1.
• The specific heat of iron is 0.450 J g1 K-"!.
Unrounded
Rounded
-1.98 x 103
qFe, ()
-1977.10
4977.1
2124.65
2.1 x 103
9water. ()
17541.3005
2124.7
-147.55
-148
ICal, ()
19548.40
4.48-103
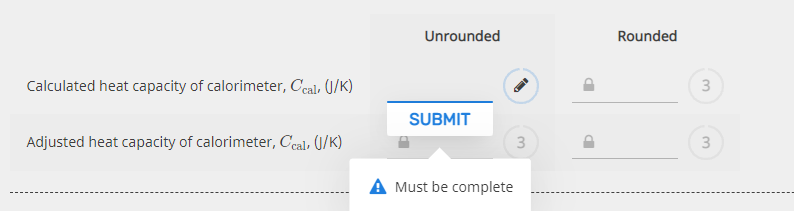
Transcribed Image Text:Unrounded
Rounded
Calculated heat capacity of calorimeter, Ccal, (1/K)
SUBMIT
Adjusted heat capacity of calorimeter, Cecal, (/K)
3
A Must be complete
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning