Calculate the partial pre (mmHg). 1 in Hg = 25.4 mmHg 1 cm water = 0.735559 mmHg 1 mol Mg = 24.305 g Mg i TABLE D-4 TEMP 1_DEG₁_C_ 0 1 2 F 250 DIN 2002 10 0.0 14.579 4.924 5.291 5.683 6.100 1 6.143 17.016 | I 7.518 I 8.051 8.617 9.219 0.1 0.2 1. 0.31 0.4 0.5 10.6 1 4.612 4.959 4.959 H2 VAPOR PRESSURE OF WATER 030 DEG. C IN MM HG 4.646 4.660 4.714 5.031 5.068 5.406 5.406 5.445 5.805 | 5.146 1 6.230 1 6.274 6.82 | 6.729 6. 826.729 4.995 5.367 | 5.329 5.723 | 5.764 | 6.186 I 6.544 1 6.589 | 6.636 4.818 4.853 | 4.888 5.178 | 5.216 | 5.253 | 5.484 5.523 5.5+3 5.602 | 5.647 5.888 | 5.9301 5.972 1 6.014 | 6.057 1 6.313 I 5.363 6.407 1 6.453 | 6.498 | 6.776 1 6.823 | 6.871 | 6.919 | 6.967 T 1 T 7.064 | 7.114 | 7.163 | 7.213 | 7.263 1 7.313 7.364 | 7.415 | 7.446 | 7.569 | 7.622 7.942 7.996 8.387 | 8.444 | A.501 8.559 7.8347.888 8.106 8.162 7.74 | 7.727 | 7.780 8.330 8.913 8.217 | 8.273 8.794 | 8.854 8.676 8.735 8.974 19.034 | 9.095 | 9.157 | 9.281 | 9.343 | 9.406 | 9.469 | 9.533 1 9.597 | 9.661 | 9.726 | 9.791 1 I 1 your answer in torr 4.783 4.748 | 4.783 5.1045.141 0.7 10.H 1 0₂2 19.856 9.922 19.989 110.055 10.122 110.189 110.257 110.376 10.394 110.463 110.533 110.602 110.673 110.743 110.814 110.886 110.958 111.030 (11.103 111.176 111.250 111.323 111.398 11.473 11.548 11.624 11.700 111.776 111.853 111.931 |12,009 |12.087 |12.166 |12.245 |12.325 |12.4C5 |12.485 ||2.567 |12.640 |12.730 112.813 112.895 112.979 13.063 113.147 113.232 113.317 113.403 113.489 113.576 1 1 I 113.663 113.751 113.839 113.928 114.017 114.107 114.197 114.288 114.379 14.471 114.563 14.656 114.749 114.843 14.937 115.032 115.128 115.223 115.320 115.417 | 15.515 115.612 115.711 15.810 15.910 16.010 16.111 116.212 116.314 116.417 116.520 116.623 116.727 116.832 116.937 117.043 117.150 117.257 117.364 117.473 1 117.581 117.691 117.801 117.911 18.023 118.134 118.247 118.360 118.473 118.587 T T T I 118.702 118.818 118.934 119.050 119.168 119.286 119.404 119.524 119.643 119.764 119.885 120.007 120.130 120.252 120.376 120.501 120.626 120.752 120.878 121.005 121.133 121.261 121.390 121.520 121.651_121.782_121.914 122.046 122.179 122.313 122.448 122.583 122.720 122.856 122.994 123.132 123.271 173.411 123.551 123.602 123.834 123.977 124.120 124.264 124.409 124.555 124.701 124.848 124.996 125.145 T 1 125.295 125.445 125.596 125.747 125.900 126.053 126.207 126.362 126.518 126.674 126.832 126.990 127.149 127.308 127.469 127.630 127.792 127.956 178.119 128.2P4 | 128.450 128.616 128.783 128.951 129.120 129.290 129.460 129.632 129.804 129.978 1 130.152, 130.326 130.503 130.679 130.857 131.035 131.215 131.395 131.576 131.758 130 131.942 132.125 132.310 132.496 132.683 132.070 133.059 133.248 133.439 133.630-1 26 Sep 85 ETL 1110-2-253 A student completes the experiment The Universal Gas Constant and obtains the following data for one trial. 80 F3 mass of magnesium (g): Initial gas volume (ml): Final Volume (mL): Temperature (°C): Atmospheric pressure (inHg): Ah (cm of water): ů Calculate the partial pressure of hydrogen, PH2, for this trial. Give your answer in torr (mmHg). TABLE D-4 1 in Hg = 25.4 mmHg 1 cm water = 0.735559 mmHg 1 mol Mg = 24.305 g Mg . TEMP I 0.0 1_DEGA_C_1 3 I 1 T F4 I Q 0 1 2 3 4 5 6 9 7 8 9 10 12 13 15 0.1 0.0357 0.00 31.12 21.3 30.59 I 6.100 1 6.143 16.544 1 6.589 12.98 0 F5 0.2 I 4.579 | 4.612 4.646 4.660 4.9244.959 4.995 5.031 15.291 5.329 5.367 5.406 5.445 15.683 15.723 | 5.7645.805 15.46 6.186 6.230 16.274 VAPOR PRESSURE OF WATER 0 30 DEG. C IN MM HG 0.3 1 0,4 10,5 10.6 5.888 5.930 5.972 16.0141 6.057 1 1 6.318 15.363 6.407 16.453 6.498 1 6.636 | 6.92 | 6.729 6.776 6.823 | 6.871 | 6.9191 6.967 1 T 1 1 1 T 1 1 7,016 | 7.064 L 714 L_7.101 L 1,213 | 1263 | 7,313 7364 LT.415 | 7,nh I 7.518 I 7.569 18.051 18.617 9.219 1 7.8347.888 7.942 7.996 8.106 8.387 | 8.444 | .501 18.559 8.676 8.974 19.034 19.095 | 9.157 1 9.281 9.597 4.661 9.776 19.791 1 I T 9.856 9.922 9.989 10.055 10.122 110.189 110.257 110.376 10.394 110.463 | 1 110.533 110.602 110.673 110.743 10.814 110.886 110.958 111.030 111.103 111.176 1 111.250 111.323 111.398 111.473 11.548 111.624 111.700 111.776 111.853 111.931 1 |T2,009 |12.087 |12.166 |12.245 |12.325 |12.405 |12.485 |12.567 |12.640 |12.730 | 112.813 112.895 112.979 113.063 113.147 113.232 113.317 113.403 113.489 113.576 1 7.622 7.674 | 7.727 7.780 8.162 | 8.217 | 8.273 | 8.330 8.735 I 8.7948.854 8.913 9.343 9.406 | 9.469 | 9.533 1 I MUN 1 F6 0.7 1 0.10.2 4.714 | 4.748 | 4.783 4.818 4.853 14.888 1 5.068 5.104 | 5.14115.178 | 5.216 | 5.253 | 5.484 5.523 5.563 5.602 5.647 | F7 DII F8 F9 F10
Calculate the partial pre (mmHg). 1 in Hg = 25.4 mmHg 1 cm water = 0.735559 mmHg 1 mol Mg = 24.305 g Mg i TABLE D-4 TEMP 1_DEG₁_C_ 0 1 2 F 250 DIN 2002 10 0.0 14.579 4.924 5.291 5.683 6.100 1 6.143 17.016 | I 7.518 I 8.051 8.617 9.219 0.1 0.2 1. 0.31 0.4 0.5 10.6 1 4.612 4.959 4.959 H2 VAPOR PRESSURE OF WATER 030 DEG. C IN MM HG 4.646 4.660 4.714 5.031 5.068 5.406 5.406 5.445 5.805 | 5.146 1 6.230 1 6.274 6.82 | 6.729 6. 826.729 4.995 5.367 | 5.329 5.723 | 5.764 | 6.186 I 6.544 1 6.589 | 6.636 4.818 4.853 | 4.888 5.178 | 5.216 | 5.253 | 5.484 5.523 5.5+3 5.602 | 5.647 5.888 | 5.9301 5.972 1 6.014 | 6.057 1 6.313 I 5.363 6.407 1 6.453 | 6.498 | 6.776 1 6.823 | 6.871 | 6.919 | 6.967 T 1 T 7.064 | 7.114 | 7.163 | 7.213 | 7.263 1 7.313 7.364 | 7.415 | 7.446 | 7.569 | 7.622 7.942 7.996 8.387 | 8.444 | A.501 8.559 7.8347.888 8.106 8.162 7.74 | 7.727 | 7.780 8.330 8.913 8.217 | 8.273 8.794 | 8.854 8.676 8.735 8.974 19.034 | 9.095 | 9.157 | 9.281 | 9.343 | 9.406 | 9.469 | 9.533 1 9.597 | 9.661 | 9.726 | 9.791 1 I 1 your answer in torr 4.783 4.748 | 4.783 5.1045.141 0.7 10.H 1 0₂2 19.856 9.922 19.989 110.055 10.122 110.189 110.257 110.376 10.394 110.463 110.533 110.602 110.673 110.743 110.814 110.886 110.958 111.030 (11.103 111.176 111.250 111.323 111.398 11.473 11.548 11.624 11.700 111.776 111.853 111.931 |12,009 |12.087 |12.166 |12.245 |12.325 |12.4C5 |12.485 ||2.567 |12.640 |12.730 112.813 112.895 112.979 13.063 113.147 113.232 113.317 113.403 113.489 113.576 1 1 I 113.663 113.751 113.839 113.928 114.017 114.107 114.197 114.288 114.379 14.471 114.563 14.656 114.749 114.843 14.937 115.032 115.128 115.223 115.320 115.417 | 15.515 115.612 115.711 15.810 15.910 16.010 16.111 116.212 116.314 116.417 116.520 116.623 116.727 116.832 116.937 117.043 117.150 117.257 117.364 117.473 1 117.581 117.691 117.801 117.911 18.023 118.134 118.247 118.360 118.473 118.587 T T T I 118.702 118.818 118.934 119.050 119.168 119.286 119.404 119.524 119.643 119.764 119.885 120.007 120.130 120.252 120.376 120.501 120.626 120.752 120.878 121.005 121.133 121.261 121.390 121.520 121.651_121.782_121.914 122.046 122.179 122.313 122.448 122.583 122.720 122.856 122.994 123.132 123.271 173.411 123.551 123.602 123.834 123.977 124.120 124.264 124.409 124.555 124.701 124.848 124.996 125.145 T 1 125.295 125.445 125.596 125.747 125.900 126.053 126.207 126.362 126.518 126.674 126.832 126.990 127.149 127.308 127.469 127.630 127.792 127.956 178.119 128.2P4 | 128.450 128.616 128.783 128.951 129.120 129.290 129.460 129.632 129.804 129.978 1 130.152, 130.326 130.503 130.679 130.857 131.035 131.215 131.395 131.576 131.758 130 131.942 132.125 132.310 132.496 132.683 132.070 133.059 133.248 133.439 133.630-1 26 Sep 85 ETL 1110-2-253 A student completes the experiment The Universal Gas Constant and obtains the following data for one trial. 80 F3 mass of magnesium (g): Initial gas volume (ml): Final Volume (mL): Temperature (°C): Atmospheric pressure (inHg): Ah (cm of water): ů Calculate the partial pressure of hydrogen, PH2, for this trial. Give your answer in torr (mmHg). TABLE D-4 1 in Hg = 25.4 mmHg 1 cm water = 0.735559 mmHg 1 mol Mg = 24.305 g Mg . TEMP I 0.0 1_DEGA_C_1 3 I 1 T F4 I Q 0 1 2 3 4 5 6 9 7 8 9 10 12 13 15 0.1 0.0357 0.00 31.12 21.3 30.59 I 6.100 1 6.143 16.544 1 6.589 12.98 0 F5 0.2 I 4.579 | 4.612 4.646 4.660 4.9244.959 4.995 5.031 15.291 5.329 5.367 5.406 5.445 15.683 15.723 | 5.7645.805 15.46 6.186 6.230 16.274 VAPOR PRESSURE OF WATER 0 30 DEG. C IN MM HG 0.3 1 0,4 10,5 10.6 5.888 5.930 5.972 16.0141 6.057 1 1 6.318 15.363 6.407 16.453 6.498 1 6.636 | 6.92 | 6.729 6.776 6.823 | 6.871 | 6.9191 6.967 1 T 1 1 1 T 1 1 7,016 | 7.064 L 714 L_7.101 L 1,213 | 1263 | 7,313 7364 LT.415 | 7,nh I 7.518 I 7.569 18.051 18.617 9.219 1 7.8347.888 7.942 7.996 8.106 8.387 | 8.444 | .501 18.559 8.676 8.974 19.034 19.095 | 9.157 1 9.281 9.597 4.661 9.776 19.791 1 I T 9.856 9.922 9.989 10.055 10.122 110.189 110.257 110.376 10.394 110.463 | 1 110.533 110.602 110.673 110.743 10.814 110.886 110.958 111.030 111.103 111.176 1 111.250 111.323 111.398 111.473 11.548 111.624 111.700 111.776 111.853 111.931 1 |T2,009 |12.087 |12.166 |12.245 |12.325 |12.405 |12.485 |12.567 |12.640 |12.730 | 112.813 112.895 112.979 113.063 113.147 113.232 113.317 113.403 113.489 113.576 1 7.622 7.674 | 7.727 7.780 8.162 | 8.217 | 8.273 | 8.330 8.735 I 8.7948.854 8.913 9.343 9.406 | 9.469 | 9.533 1 I MUN 1 F6 0.7 1 0.10.2 4.714 | 4.748 | 4.783 4.818 4.853 14.888 1 5.068 5.104 | 5.14115.178 | 5.216 | 5.253 | 5.484 5.523 5.563 5.602 5.647 | F7 DII F8 F9 F10
Oh no! Our experts couldn't answer your question.
Don't worry! We won't leave you hanging. Plus, we're giving you back one question for the inconvenience.
Submit your question and receive a step-by-step explanation from our experts in as fast as 30 minutes.
You have no more questions left.
Message from our expert:
Writing assignments don't fall within the 30+ subjects covered in Ask an Expert. For writing help, please visit bartleby write. We've credited a question to your account.
Your Question:
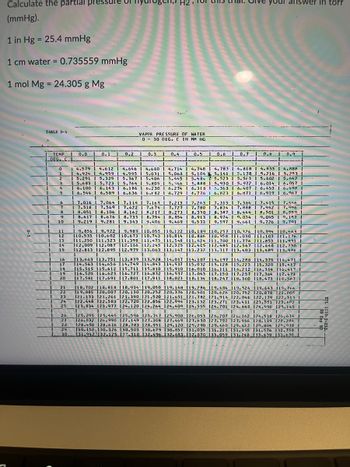
Transcribed Image Text:Calculate the partial pre
(mmHg).
1 in Hg = 25.4 mmHg
1 cm water = 0.735559 mmHg
1 mol Mg = 24.305 g Mg
i
TABLE D-4
TEMP
1_DEG₁_C_
0
1
2
F 250 DIN 2002
10
0.0
14.579
4.924
5.291
5.683
6.100 1 6.143
17.016 |
I 7.518 I
8.051
8.617
9.219
0.1 0.2 1. 0.31 0.4 0.5 10.6
1
4.612
4.959
4.959
H2
VAPOR PRESSURE OF WATER
030 DEG. C IN MM HG
4.646 4.660 4.714
5.031 5.068
5.406
5.406 5.445
5.805 | 5.146
1
6.230 1 6.274
6.82 | 6.729
6. 826.729
4.995
5.367 |
5.329
5.723 | 5.764 |
6.186
I 6.544 1 6.589 | 6.636
4.818 4.853 | 4.888
5.178 | 5.216 | 5.253 |
5.484 5.523 5.5+3 5.602 | 5.647
5.888 | 5.9301 5.972 1 6.014 | 6.057 1
6.313 I 5.363 6.407 1 6.453 | 6.498
| 6.776 1 6.823 | 6.871 | 6.919 | 6.967
T
1
T
7.064 | 7.114 | 7.163 | 7.213 | 7.263 1 7.313 7.364 | 7.415 | 7.446 |
7.569 | 7.622
7.942 7.996
8.387 | 8.444 | A.501 8.559
7.8347.888
8.106 8.162
7.74 | 7.727 | 7.780
8.330
8.913
8.217 | 8.273
8.794 | 8.854
8.676 8.735
8.974 19.034 | 9.095 | 9.157 |
9.281 | 9.343 | 9.406 | 9.469 | 9.533 1 9.597 | 9.661 | 9.726 | 9.791
1
I
1
your answer in torr
4.783
4.748 | 4.783
5.1045.141
0.7 10.H 1 0₂2
19.856 9.922 19.989 110.055 10.122 110.189 110.257 110.376 10.394 110.463
110.533 110.602 110.673 110.743 110.814 110.886 110.958 111.030 (11.103 111.176
111.250 111.323 111.398 11.473 11.548 11.624 11.700 111.776 111.853 111.931
|12,009 |12.087 |12.166 |12.245 |12.325 |12.4C5 |12.485 ||2.567 |12.640 |12.730
112.813 112.895 112.979 13.063 113.147 113.232 113.317 113.403 113.489 113.576
1
1
I
113.663 113.751 113.839 113.928 114.017 114.107 114.197 114.288 114.379 14.471
114.563 14.656 114.749 114.843 14.937 115.032 115.128 115.223 115.320 115.417 |
15.515 115.612 115.711 15.810 15.910 16.010 16.111 116.212 116.314 116.417
116.520 116.623 116.727 116.832 116.937 117.043 117.150 117.257 117.364 117.473 1
117.581 117.691 117.801 117.911 18.023 118.134 118.247 118.360 118.473 118.587
T
T
T
I
118.702 118.818 118.934 119.050 119.168 119.286 119.404 119.524 119.643 119.764
119.885 120.007 120.130 120.252 120.376 120.501 120.626 120.752 120.878 121.005
121.133 121.261 121.390 121.520 121.651_121.782_121.914 122.046 122.179 122.313
122.448 122.583 122.720 122.856 122.994 123.132 123.271 173.411 123.551 123.602
123.834 123.977 124.120 124.264 124.409 124.555 124.701 124.848 124.996 125.145
T
1
125.295 125.445 125.596 125.747 125.900 126.053 126.207 126.362 126.518 126.674
126.832 126.990 127.149 127.308 127.469 127.630 127.792 127.956 178.119 128.2P4 |
128.450 128.616 128.783 128.951 129.120 129.290 129.460 129.632 129.804 129.978 1
130.152, 130.326 130.503 130.679 130.857 131.035 131.215 131.395 131.576 131.758
130 131.942 132.125 132.310 132.496 132.683 132.070 133.059 133.248 133.439 133.630-1
26 Sep 85
ETL 1110-2-253
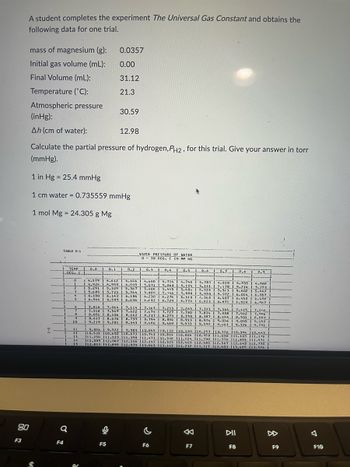
Transcribed Image Text:A student completes the experiment The Universal Gas Constant and obtains the
following data for one trial.
80
F3
mass of magnesium (g):
Initial gas volume (ml):
Final Volume (mL):
Temperature (°C):
Atmospheric pressure
(inHg):
Ah (cm of water):
ů
Calculate the partial pressure of hydrogen, PH2, for this trial. Give your answer in torr
(mmHg).
TABLE D-4
1 in Hg = 25.4 mmHg
1 cm water = 0.735559 mmHg
1 mol Mg = 24.305 g Mg
.
TEMP I 0.0
1_DEGA_C_1
3
I
1
T
F4
I
Q
0
1
2
3
4
5
6
9
7
8
9
10
12
13
15
0.1
0.0357
0.00
31.12
21.3
30.59
I 6.100 1 6.143
16.544 1 6.589
12.98
0
F5
0.2
I 4.579 | 4.612 4.646 4.660
4.9244.959 4.995 5.031
15.291 5.329 5.367 5.406 5.445
15.683 15.723 | 5.7645.805 15.46
6.186 6.230 16.274
VAPOR PRESSURE OF WATER
0 30 DEG. C IN MM HG
0.3 1 0,4 10,5 10.6
5.888 5.930 5.972 16.0141 6.057 1
1
6.318 15.363 6.407 16.453 6.498 1
6.636 | 6.92 | 6.729 6.776 6.823 | 6.871 | 6.9191 6.967 1
T
1
1
1
T
1
1 7,016 | 7.064 L 714 L_7.101 L 1,213 | 1263 | 7,313 7364 LT.415 | 7,nh
I 7.518 I 7.569
18.051
18.617
9.219
1
7.8347.888 7.942 7.996
8.106
8.387 | 8.444 | .501 18.559
8.676
8.974 19.034 19.095 | 9.157 1
9.281
9.597 4.661 9.776 19.791 1
I
T
9.856 9.922 9.989 10.055 10.122 110.189 110.257 110.376 10.394 110.463 |
1
110.533 110.602 110.673 110.743 10.814 110.886 110.958 111.030 111.103 111.176 1
111.250 111.323 111.398 111.473 11.548 111.624 111.700 111.776 111.853 111.931 1
|T2,009 |12.087 |12.166 |12.245 |12.325
|12.405 |12.485 |12.567 |12.640 |12.730 |
112.813 112.895 112.979 113.063 113.147 113.232 113.317 113.403 113.489 113.576 1
7.622 7.674 | 7.727 7.780
8.162 | 8.217 | 8.273 | 8.330
8.735 I 8.7948.854 8.913
9.343 9.406 | 9.469 | 9.533
1
I
MUN 1
F6
0.7 1 0.10.2
4.714 | 4.748 | 4.783 4.818
4.853 14.888 1
5.068 5.104 | 5.14115.178 | 5.216 | 5.253 |
5.484 5.523 5.563 5.602 5.647 |
F7
DII
F8
F9
F10
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

