Mass of Mass of Mass of 'process (kJ/mol) Salt Used Salt (g) Calorimeter Calorimeter T, (°C) T; (°C) (g) + H,0 (g) CaCl2 6.3307 2.3245 44.8883 23.5 KCL 6.3357 44.8884 tebook 2.3312
Mass of Mass of Mass of 'process (kJ/mol) Salt Used Salt (g) Calorimeter Calorimeter T, (°C) T; (°C) (g) + H,0 (g) CaCl2 6.3307 2.3245 44.8883 23.5 KCL 6.3357 44.8884 tebook 2.3312
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 26E: When 50.0 g of 0.200 M NaCl(aq) at 24.1 C is added to 100.0 g of 0.100 M AgNO3(aq) at 24.1 C in a...
Related questions
Question
q= Cp× m× ∆T
I need help to find H Process in kJ/mol.
last column for both cacl2 and KCL
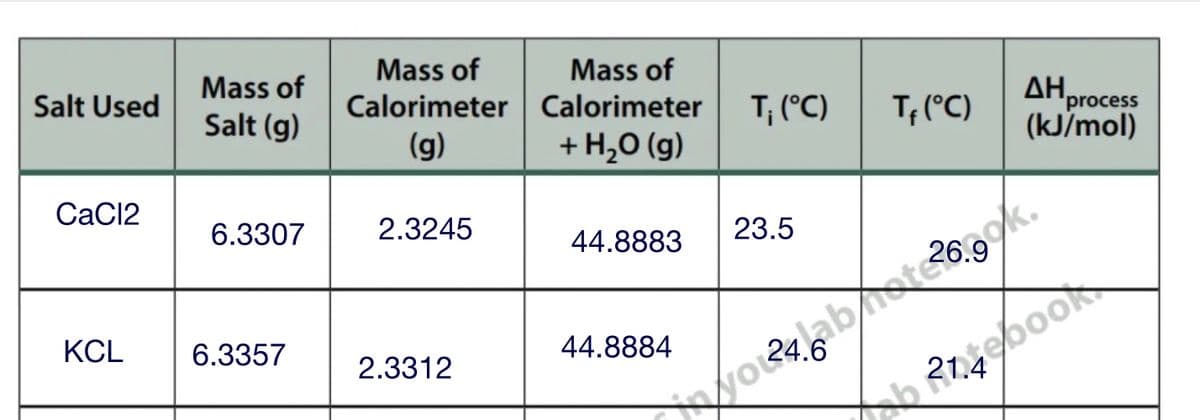
Transcribed Image Text:Mass of
Calorimeter Calorimeter
Mass of
Mass of
Salt Used
T; (°C)
ΔΗ
'process
(kJ/mol)
Salt (g)
T; (°C)
(g)
+ H,O (g)
CaC12
iyoab/steook.
Rtebook
6.3307
2.3245
23.5
44.8883
KCL
6.3357
44.8884
2.3312
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning