NaOH(s) trial (A) NHẠNO3 (s) trial (B) Part 2: Enthalpy of dissolution a. Mass of substance dissolved (g) b. Mass of calorimeter (g) c. Mass of calorimeter + water (g) d. Mass of water (g) 0.8314 0.7985 2.114 2.179 11.9717 12.0731 e. Total mass of reactants (g) f. Initial temperature of water (°C) g. Final temperature of solution (°C) h. Change in the temperature, AT (°C) i. Moles of solid dissolved in water (mols) j. Heat lost or gained by the water, Aqm (J) k. Heat absorbed by the calorimeter, Aq. (J) I. Heat absorbed or released by the dissolving solute Aq: = Aqm+ Aqc (J) m. Enthalpy of solution, AHrxn (kJ/mol) n. Theoretical enthalpy, calculated AHrxn (kJ/mol) 18.0 18.2 30.0 13.6 o. Percent error
NaOH(s) trial (A) NHẠNO3 (s) trial (B) Part 2: Enthalpy of dissolution a. Mass of substance dissolved (g) b. Mass of calorimeter (g) c. Mass of calorimeter + water (g) d. Mass of water (g) 0.8314 0.7985 2.114 2.179 11.9717 12.0731 e. Total mass of reactants (g) f. Initial temperature of water (°C) g. Final temperature of solution (°C) h. Change in the temperature, AT (°C) i. Moles of solid dissolved in water (mols) j. Heat lost or gained by the water, Aqm (J) k. Heat absorbed by the calorimeter, Aq. (J) I. Heat absorbed or released by the dissolving solute Aq: = Aqm+ Aqc (J) m. Enthalpy of solution, AHrxn (kJ/mol) n. Theoretical enthalpy, calculated AHrxn (kJ/mol) 18.0 18.2 30.0 13.6 o. Percent error
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 75AP
Related questions
Question
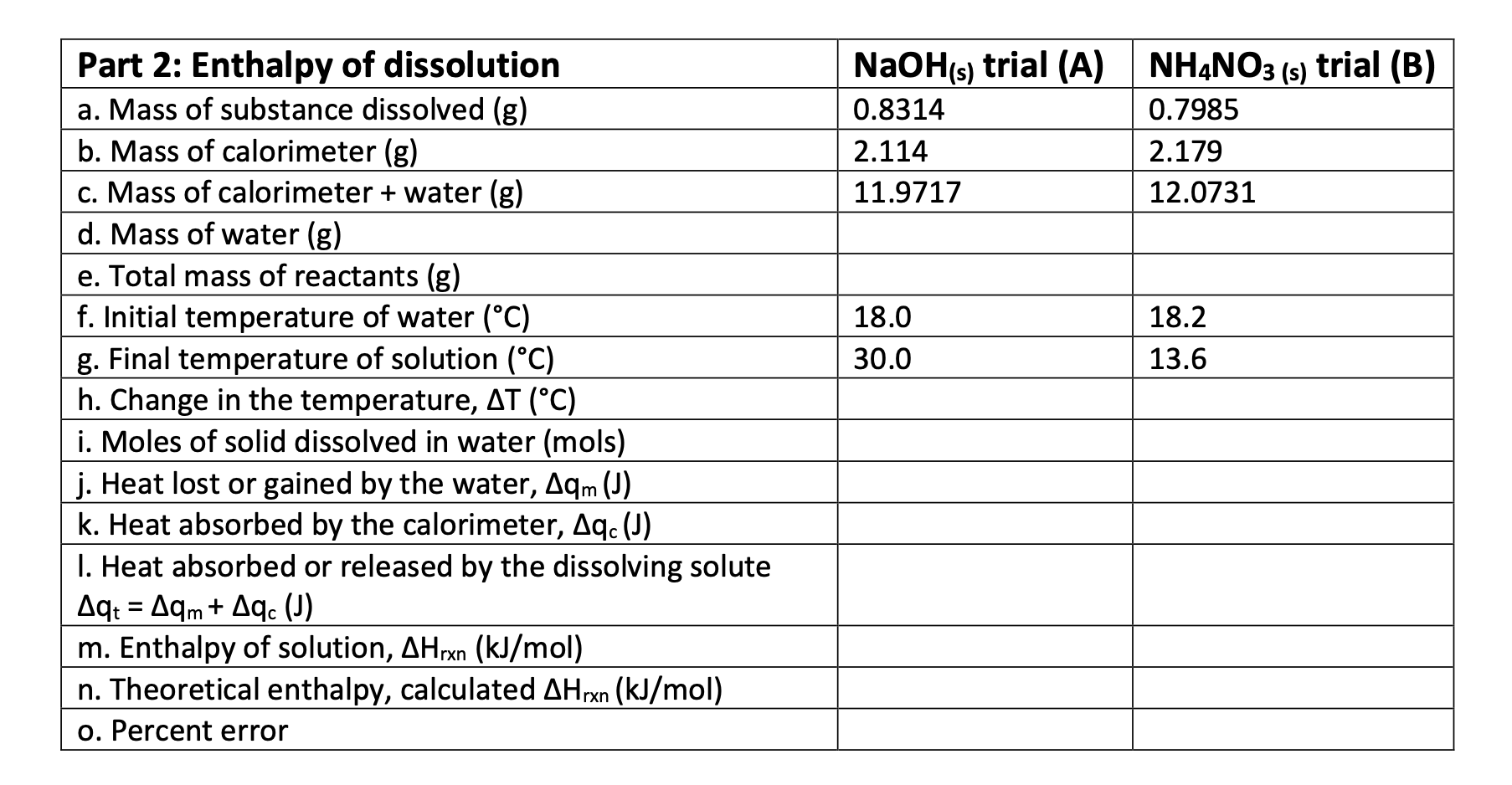
Transcribed Image Text:NaOH(s) trial (A) NHẠNO3 (s) trial (B)
Part 2: Enthalpy of dissolution
a. Mass of substance dissolved (g)
b. Mass of calorimeter (g)
c. Mass of calorimeter + water (g)
d. Mass of water (g)
0.8314
0.7985
2.114
2.179
11.9717
12.0731
e. Total mass of reactants (g)
f. Initial temperature of water (°C)
g. Final temperature of solution (°C)
h. Change in the temperature, AT (°C)
i. Moles of solid dissolved in water (mols)
j. Heat lost or gained by the water, Aqm (J)
k. Heat absorbed by the calorimeter, Aq. (J)
I. Heat absorbed or released by the dissolving solute
Aq: = Aqm+ Aqc (J)
m. Enthalpy of solution, AHrxn (kJ/mol)
n. Theoretical enthalpy, calculated AHrxn (kJ/mol)
18.0
18.2
30.0
13.6
o. Percent error
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning