mass of test tube + beaker + stir bar + solvent (g) 30.64 mass of test tube + beaker + stir bar + solvent + solute (g) 38.48 mass of test tube + beaker + stir bar (g) 10.23 mass of solvent (g) (A) 20.41 mass of solute (g) (B) 7.84 freezing point pure solvent (°C) 45.36 freezing point solution (°C) 41.56 If the freezing point depression constant of the pure solvent is 8.68 °C-kg solvent/mol solute, calculate the molar mass of the solute g/mol
mass of test tube + beaker + stir bar + solvent (g) 30.64 mass of test tube + beaker + stir bar + solvent + solute (g) 38.48 mass of test tube + beaker + stir bar (g) 10.23 mass of solvent (g) (A) 20.41 mass of solute (g) (B) 7.84 freezing point pure solvent (°C) 45.36 freezing point solution (°C) 41.56 If the freezing point depression constant of the pure solvent is 8.68 °C-kg solvent/mol solute, calculate the molar mass of the solute g/mol
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter17: Spontaneity, Entropy, And Free Energy
Section: Chapter Questions
Problem 128IP: Some nonelectrolyte solute (molar mass = 142 g/mol) was dissolved in 150. mL of a solvent (density =...
Related questions
Question
Assume van't Hoff factor is 1.00
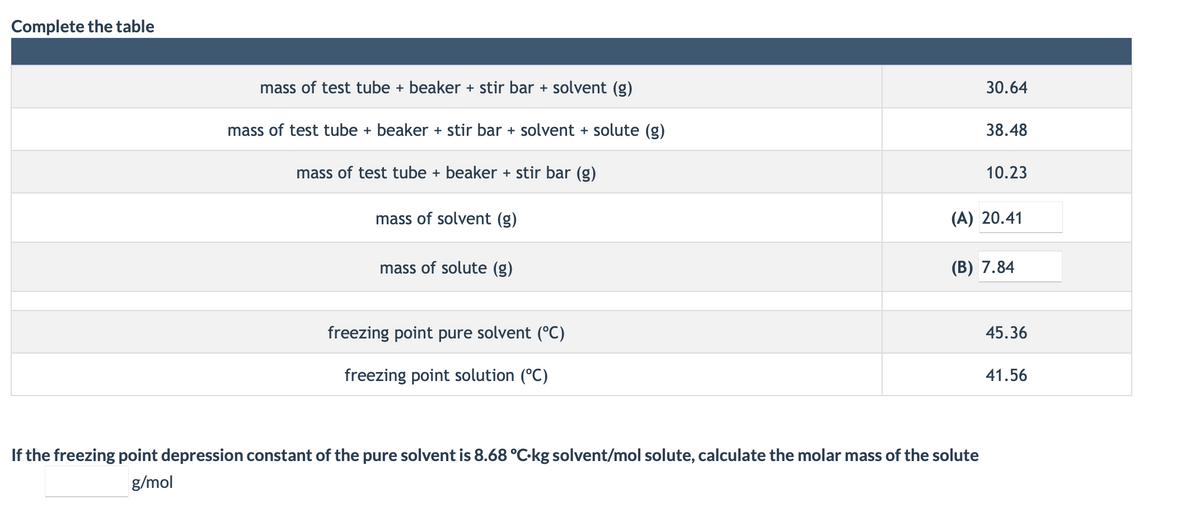
Transcribed Image Text:Complete the table
mass of test tube + beaker + stir bar + solvent (g)
30.64
mass of test tube + beaker + stir bar + solvent + solute (g)
38.48
mass of test tube + beaker + stir bar (g)
10.23
mass of solvent (g)
(A) 20.41
mass of solute (g)
(В) 7.84
freezing point pure solvent (°C)
45.36
freezing point solution (°C)
41.56
If the freezing point depression constant of the pure solvent is 8.68 °C-kg solvent/mol solute, calculate the molar mass of the solute
g/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,