Mass Spectrometry of Molecular Compounds Mass spectrometry is not only used for isotopic evaluation of the elements. It can also be used for identification of molecular samples as well. Consider, for example, the mass spectrum of pentane (Figure 5): -43 relative abundance 57 72 10 20' 30' 40 50 60 70 miz Figure 5. Pentane Mass Spectrum The pentane molecule (CH,CH,CH,CH,CH, or C;H12) is a straight-chain hydrocarbon. In Figure 5, notice there are four groups of peaks. This forms what is known as the cracking pattern of the molecule. The first peak is at m/z = 72, corresponding to the molecular ion, C3H,2", where only one electron was knocked off the pentane molecule. The three peak groups are from fragments of pentane created in the ionizer: m/z = 57 (CH,CH;CH,CH,"), m/z = 43 (CH;CH,CH;"), m/z = 29 (CH;CH;"). These species have had pieces of the molecule knocked off in addition to losing an electron. 4. For the peak at m/z = 43, what part of the pentane molecule was knocked off? Use m/z values to justify your answer.
Mass Spectrometry of Molecular Compounds Mass spectrometry is not only used for isotopic evaluation of the elements. It can also be used for identification of molecular samples as well. Consider, for example, the mass spectrum of pentane (Figure 5): -43 relative abundance 57 72 10 20' 30' 40 50 60 70 miz Figure 5. Pentane Mass Spectrum The pentane molecule (CH,CH,CH,CH,CH, or C;H12) is a straight-chain hydrocarbon. In Figure 5, notice there are four groups of peaks. This forms what is known as the cracking pattern of the molecule. The first peak is at m/z = 72, corresponding to the molecular ion, C3H,2", where only one electron was knocked off the pentane molecule. The three peak groups are from fragments of pentane created in the ionizer: m/z = 57 (CH,CH;CH,CH,"), m/z = 43 (CH;CH,CH;"), m/z = 29 (CH;CH;"). These species have had pieces of the molecule knocked off in addition to losing an electron. 4. For the peak at m/z = 43, what part of the pentane molecule was knocked off? Use m/z values to justify your answer.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter2: Atoms Molecules And Ions
Section: Chapter Questions
Problem 100PS
Related questions
Question
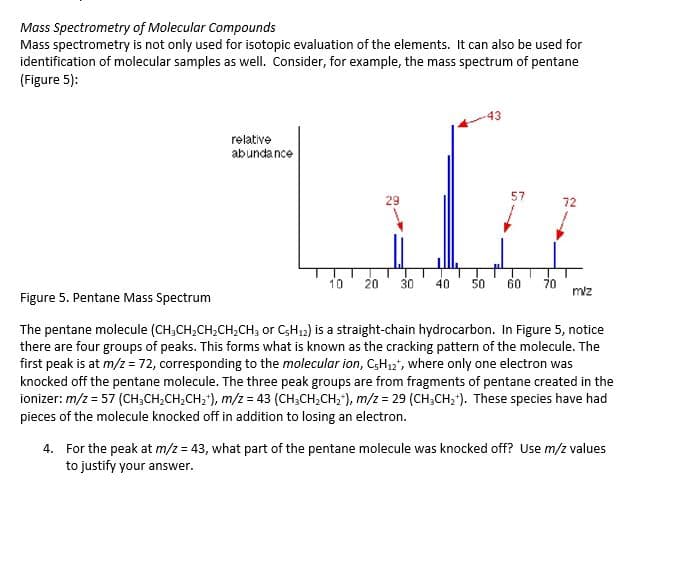
Transcribed Image Text:Mass Spectrometry of Molecular Compounds
Mass spectrometry is not only used for isotopic evaluation of the elements. It can also be used for
identification of molecular samples as well. Consider, for example, the mass spectrum of pentane
(Figure 5):
Lifie
relative
abundance
57
29
72
10
20
30
40
50
60
70
miz
Figure 5. Pentane Mass Spectrum
The pentane molecule (CH;CH,CH;CH;CH; or C;H12) is a straight-chain hydrocarbon. In Figure 5, notice
there are four groups of peaks. This forms what is known as the cracking pattern of the molecule. The
first peak is at m/z = 72, corresponding to the molecular ion, C;H,2", where only one electron was
knocked off the pentane molecule. The three peak groups are from fragments of pentane created in the
ionizer: m/z = 57 (CH;CH,CH,CH,"), m/z = 43 (CH;CH,CH;"), m/z = 29 (CH;CH,"). These species have had
pieces of the molecule knocked off in addition to losing an electron.
4. For the peak at m/z = 43, what part of the pentane molecule was knocked off? Use m/z values
to justify your answer.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning
